
Titanium is a chemical element that is used in the automotive industry and other areas.
Titanium is the name of the chemical element that has the atomic number 22 and its symbol is Ti . It is a fairly abundant metal in the crust of our planet, whose color is grayish.
The discovery of titanium was made by British scientist William Gregor in 1790 . This element stands out for its resistance to corrosion and its hardness ; That is why it is often compared to steel. Thanks to its characteristics, titanium is a metal that is used in aerospace engineering since it can tolerate the extreme temperatures found in outer space .
Its great resistance also means that titanium is used in the chemical sector due to its ability to resist the action of different kinds of acids. The arms industry , the automobile industry and jewelry also often use this metal.
Titanium characteristics
This chemical element is found in different minerals, igneous rocks and animal and plant organisms. It is usually extracted from rutile that is present in coastal areas, refining it through different processes .
Five natural isotopes with good stability have been detected. On the other hand, scientists have recognized more than a dozen radioisotopes , most of which have little stability.
It should be noted that titanium can be cast, forged and welded, in addition to being subjected to other processes. This, added to its reduced level of toxicity , makes it suitable for a wide range of applications. Australia , South Africa and Canada are among the world's largest producers of titanium oxide.
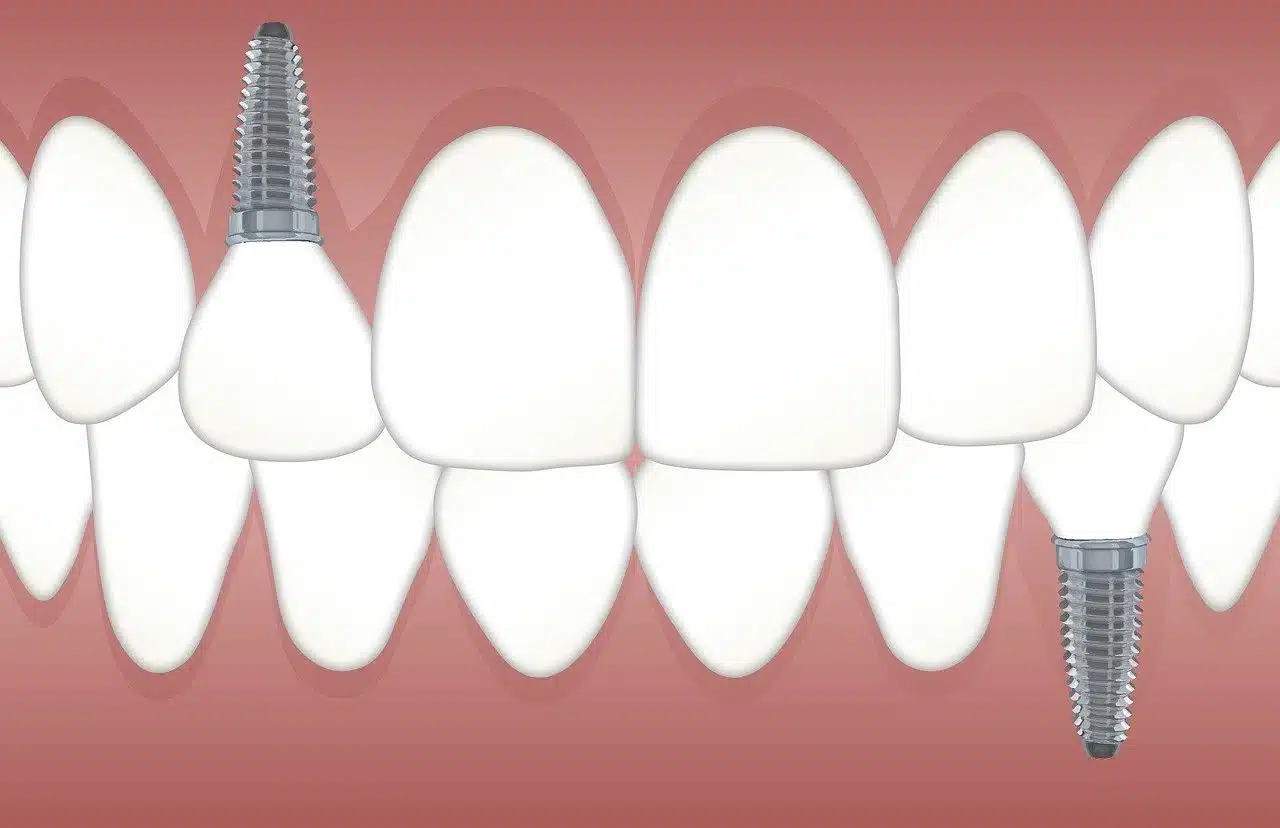
The use of titanium is common in dentistry.
Its use in dentistry
Some dental clinics choose titanium for their implants because it has a high degree of biocompatibility, and this is related to the characteristics of its surface oxide . Whether immersed in water or exposed to open air, as long as it is at room temperature, titanium quickly forms oxide with a thickness ranging from 3 to 5 nm. Among the many oxides of different stoichiometry (the calculation of the quantity relationships between products and reactants throughout a chemical reaction) that can form, the most common is TiO2 .
It is important to note that many chemical processes can take place at the interfaces of a dental implant, such as corrosion (or dissolution) of oxide, ionic transport, fragmentation and absorption of biomolecules, metabolically directed oxidation, incorporation of mineral ions. in oxide, catalytic processes and protein denaturation. Precisely, knowing this in advance, many choose titanium, because they know that it is less invasive for the body than other materials.
Titanium can have three crystal structures , and it also resists chemical attack like few other materials, something that makes it one of those that best withstands corrosion, especially in the field of orthodontics and dental implants. Another factor that makes it especially biocompatible is its high dielectric constant; This property is unique to titanium, and the value can be between 50 and 170, depending on the crystal structure, and has a positive impact on the union of osseointegrated implants (those that are fixed to the mouth and lack mobility).
In addition to its great resistance, titanium is a particularly light material, lighter than most construction materials, and this is due to its low density . For biomedical applications, it has been discovered that titanium can be alloyed very favorably with tantalum, zirconium and niobium, as long as we are talking about non-toxic materials. It is worth mentioning that, on the other hand, these alloys are not bioactive, that is, they do not bond strongly with the bones, and therefore they are not suitable for the manufacture of osseointegrated implants.
