
Reverse sublimation is known as a thermodynamic process in which a phase change occurs from the gaseous state directly to the solid state.
Reverse sublimation is, according to experts in thermodynamics , a process characterized by a phase change experienced by a substance that manages to go from the gaseous state to the solid phase, skipping the liquid state instance.
Regressive sublimation, desublimation y deposición son otras de las denominaciones que recibe este fenómeno que, por sus características, se encuadra en el grupo de los procesos exotérmicos (aquellos que al desarrollarse liberan energía). Antes de hacer hincapié en particularidades y aplicaciones de la sublimación inversa es muy importante diferenciarla bien en relación a la sublimation, donde la transition tampoco implica al estado líquido pero se parte desde un estado sólido y se llega desde ahí a la fase gaseosa.
It should be noted that inverse sublimation can be observed in any pure substance that is subjected to specific conditions of pressure and temperature levels (which are achieved in very specific natural contexts or in laboratories).
Thermodynamics of reverse sublimation
To link thermodynamics with the reverse sublimation process, it is first convenient to know (or remember) several concepts.
It is necessary to be clear, for example, that the portion of something isolated to study it is classified as a working substance or thermodynamic system . If the level of isolation is taken into account, it is possible to recognize between isolated system , closed system and open system . One can even speak of heterogeneous systems and homogeneous systems . In this framework, the equation of state becomes relevant, a resource that marks the link or connection between issues related to a hydrostatic system (internal energy, pressure, density, temperature and volume, among other variables that serve to describe the state of aggregation of the subject).
Also, for a better understanding of the phenomena and to minimize the chances of confusion, we must look at the differences that endothermic processes present (in which an increase in the thermodynamic magnitude known as enthalpy or internal energy is observed). , noticing in them the absorption of thermal energy from the environment) and exothermic processes (where the release of energy by the system can occur in the form of sound, light, electricity or heat).
And after mentioning enthalpy (which is equivalent to the sum of the internal energy of a body by adding the product obtained from the volume multiplied by the external pressure), it is worth learning about another thermodynamic quantity called entropy . The latter serves to measure the portion of energy that is not used when carrying out a job. It is expressed as the quotient arising from the heat given off by a certain body and its absolute temperature.
No less transcendent are the laws of thermodynamics ( Law of conservation of energy , Law of Entropy , Zero principle of thermodynamics ) or latent heat (as it is named how much energy a specific substance needs to achieve a change in temperature). phase , either from liquid to gaseous by the vaporization process or from solid to liquid with heat linked to fusion .

Latent heat arises from the process known as condensation (when a substance originally in a gaseous state changes to a liquid state).
Experimental methods
There are several experimental methods to learn about reverse sublimation and carefully study this process.
A valid option is calorimetry intended to measure the amount of heat that is transferred from, or to, a certain substance. Using spectroscopy (a technique that is based on the decomposition of light and calculating different wavelengths, both visible and non-visible light), on the other hand, it is possible to establish the level of light ejected, reflected or absorbed. for an element.
Likewise, methods of vapor pressure measurement and electron microscopy are useful.
Factors that influence reverse sublimation
It should be noted that there are multiple factors that influence reverse sublimation . Temperature , pressure and volume lead the list.
When faced with a closed system exposed to a certain temperature , meanwhile, a pressure is noted, known as vapor pressure , exerted by vapor or gas phase around the liquid state. This happens whenever there is a dynamic equilibrium between the vapor and the liquid phase.
And we must not ignore phase equilibrium , which implies the contact of at least two states of matter (solid, liquid or gaseous) and is determined when the chemical potential of some element that makes up the analyzed system achieves stability after a period of time. time.
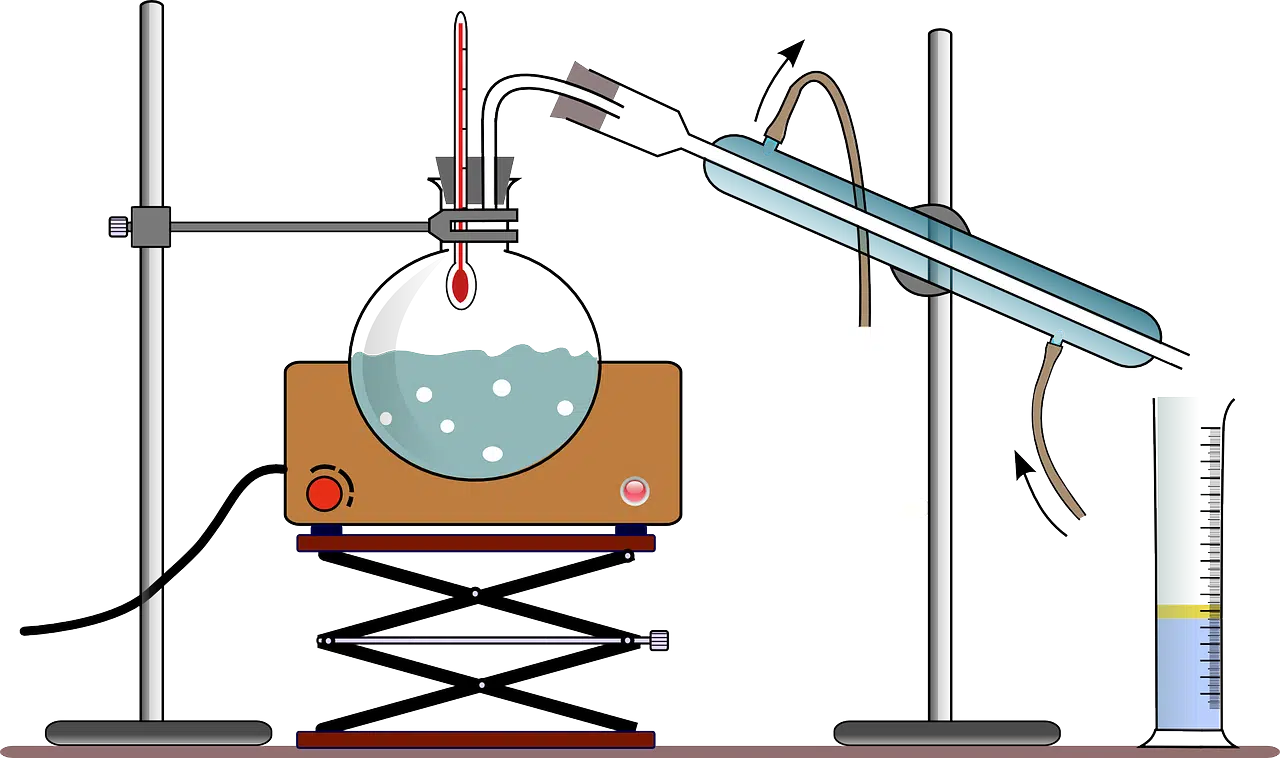
To study reverse sublimation there are several techniques and methods to apply in a laboratory.
Processes that involve a phase change
The processes that involve a phase change are five.
When sublimation takes place, the solid state is left behind and it passes directly into the gas phase . In a fusion , to describe another possibility, a substance transitions from the solid state to becoming a liquid.
Changing a substance from a liquid to a gaseous state is achieved with vaporization , while solidification is characterized by generating the evolution of a gas or a liquid towards the solid state .
Before concluding this informative article, it is interesting to refer to condensation , which is the name of a physical process during which a substance, accompanied by a special temperature and pressure, manages to change to a liquid state from an initial gaseous phase .
