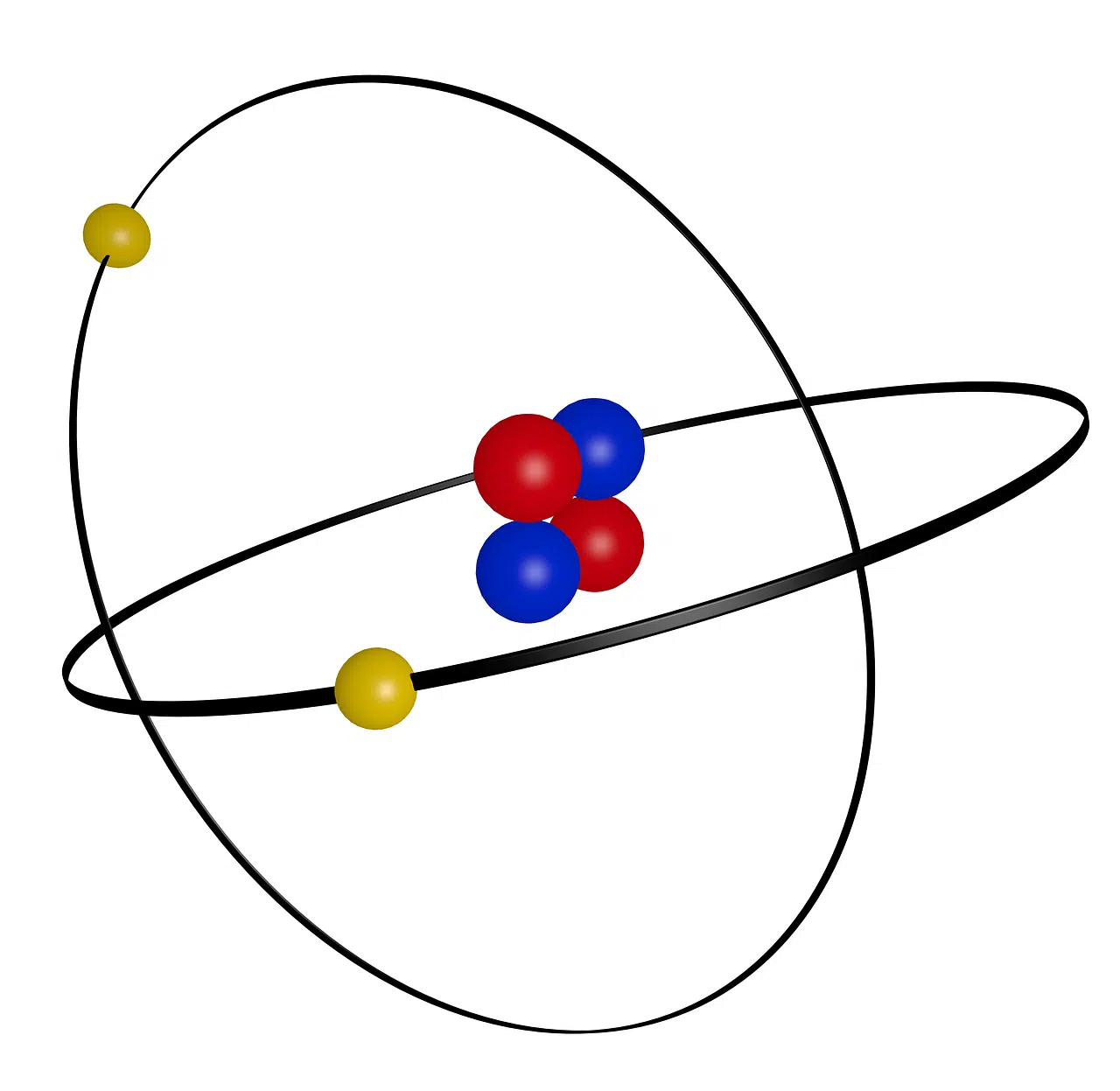
Alpha rays are formed by alpha particles (ionized helium nuclei).
Alpha rays are radiation from alpha particles . They have a very reduced penetration capacity since their mass and charge lead them to engage in interactions with other molecules.
Before moving forward, it is interesting to establish the etymological origin of the concept:
- Ray derives from the Latin radius , which can be translated as "rod" .
- Alpha comes from the Greek alpha , which is the name of the first letter of the alphabet of that language.
What are alpha rays
There are various types of rays (energy lines that originate at a point and propagate in a certain direction). We can speak, depending on the case, of light rays , X-rays , gamma rays or infrared rays , to name a few possibilities. Alpha rays , as we already indicated, are formed with alpha-type particles .
The fully ionized nucleus of helium , which originates from certain nuclear reactions or disintegration, is known as an alpha particle . These nuclei are made up of two neutrons and two protons: therefore, they do not have electrons surrounding them. Due to this characteristic, alpha particles have a mass of 4 amu and a positive charge.
Due to this limited penetration capacity, alpha rays can be stopped with some papers and are not able to penetrate the skin . Given this low penetrating power, alpha rays are not too dangerous: this situation, however, changes if the particles that are responsible for the radioactive emission are already inside the organism.

Alpha rays are named after the first letter of the Greek alphabet.
Other information of interest
In addition to everything stated above, it is worth knowing other interesting data related to alpha rays, among which these stand out:
- As a general rule, they take center stage and appear both in a nuclear reaction and in the radioactive disintegration processes of nuclides, in which they are transmuted into other elements that have the particularity of being much lighter.
- The essence, functionality and existence of these rays was demonstrated scientifically by Ernest Rutherford in 1909 .
- Smoke detection and powering batteries and various equipment are some of the uses of alpha rays.
- When we talk about alpha rays, we always also refer to beta rays . However, they are different because the latter have the peculiarity that they have a greater penetrating power.
- In the same way, the existence of gamma rays should not be overlooked either. These are identified because in their radiation there is no loss of identity on the part of the nucleus.
