 Plutonium is a radioactive substance that is characterized by its high level of toxicity . The term comes from the English word plutonium and its name is linked to the arrangement of the chemical elements in the periodic table: plutonium follows uranium and neptunium , just as Pluto follows the planets Uranus and Neptune .
Plutonium is a radioactive substance that is characterized by its high level of toxicity . The term comes from the English word plutonium and its name is linked to the arrangement of the chemical elements in the periodic table: plutonium follows uranium and neptunium , just as Pluto follows the planets Uranus and Neptune .
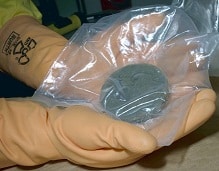
With atomic number 94 , it is a metallic chemical element that belongs to the group of actinides . Its symbol is Pu and it has highly stable isotopes , which are used as fuel and as explosives .
In the 1930s , Italian chemist Enrico Fermi believed he had discovered a new element, which he named hesperium . However, it was actually a combination of krypton , barium , and other components. Based on Fermi 's findings, research progressed and finally the production and isolation of plutonium was achieved for the first time in 1940 thanks to a scientific team from the University of California at Berkeley ( United States ).
The most stable isotopes of plutonium are plutonium-239 (which takes 24,110 years to half-decay) and plutonium-242 (whose decay time reaches 373,300 years). Although it is considered the most dangerous nuclear fuel , plutonium is used in different ways.
The manufacture of atomic bombs , the development of thermoelectric plants and the production of pacemakers are some of the uses given to plutonium. It is important to keep in mind, however, that plutonium is very harmful to all living beings , so its handling must be extremely careful.
It is very common for the media to refer to plutonium as the most toxic substance that human beings have ever discovered; Although experts point out that this is not accurate, they also do not deny its negative effects on our health . The chemical element called radium , for example, can be about two hundred times more toxic than plutonium; Its atomic number is 88, it is radioactive and rare, since its average natural occurrence is three million less than that of uranium .
 Botulinum toxin , on the other hand, is one of the organic toxins that exceeds the toxicity of plutonium in colossal proportions. Returning to plutonium, its alpha radiation cannot penetrate the skin, although if ingested or inhaled it can affect internal organs. It is enough to enter a few microscopic particles of this element to put us at risk of suffering from lung cancer.
Botulinum toxin , on the other hand, is one of the organic toxins that exceeds the toxicity of plutonium in colossal proportions. Returning to plutonium, its alpha radiation cannot penetrate the skin, although if ingested or inhaled it can affect internal organs. It is enough to enter a few microscopic particles of this element to put us at risk of suffering from lung cancer.
If the amount of plutonium that enters our body is considerable, acute poisoning or even death may occur. Curiously, statistical data does not contribute to raising awareness of the dangers of this element, since there are no cases that indicate it as the cause of death, or they have not become sufficiently publicized to attract attention.
Since plutonium has long been used in the creation of explosives and nuclear weapons, it is not uncommon for it to be released into the atmosphere in the context of certain tests or due to accidents in factories. As with other forms of contamination , plutonium does not leave the Earth forever, but rather returns to the ground after its release, and in this way its negative action expands.
It should be noted that most of the plutonium that exists today comes from production in nuclear reactors, as it naturally occurs in much smaller quantities. After an accidental release in a test, it rises into the atmosphere and can return through radioactive fallout, penetrating the soil and reaching groundwater.
