Atomic weight is a magnitude.
The force developed by the planet to attract a body and the magnitude of said force are called weight . The concept is also used as a synonym for mass (which, in reality, is the amount of matter that a body houses, regardless of the force of gravity).
Atomic , for its part, is an adjective that refers to what is linked to the atom . Atoms are the smallest indivisible particles that have autonomous existence.
What is atomic weight
These definitions help us understand the idea of atomic weight , a quantity that reveals the link between the mass of an atom of a specific class of isotope and 1/12 of the mass of a carbon-12 atom.
This magnitude, which is also known as relative atomic mass , is expressed in atomic mass units . It is important to differentiate between the relative atomic mass or atomic weight and the atomic mass (which refers to the mass of each individual atom).
Assignment of a number
What atomic weight allows is to assign a number to chemical elements to indicate what mass their atoms have on average. The atomic weight will be determined according to the proportions of the isotopes, since the same chemical element can have more than one type of isotope.
An atomic weight can be calculated from the known value of the atomic mass and according to the composition of the isotopes. The result of these operations is usually reflected in the so-called periodic table of the elements.
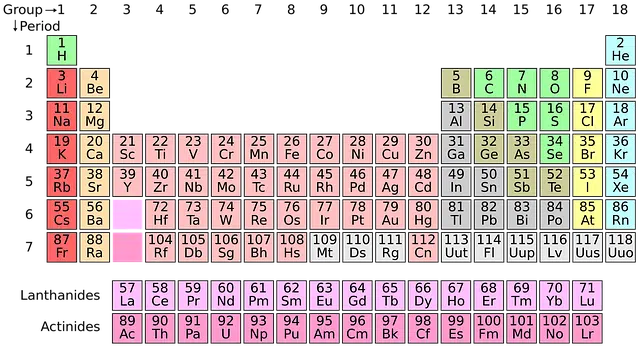
The atomic weight of the elements is usually indicated in the periodic table.
Atomic weight, a controversial concept
It is worth mentioning that in the scientific field, the use of the name "atomic weight" has been especially controversial . Scientists generally prefer to refer to this concept as "relative atomic mass" which, as mentioned above, should not be confused with atomic mass . To justify this decision, they explain that this property is not a weight but the force that, in a gravitational field, is exerted on a given object and that is measured in some unit of force, such as the newton .
On the other hand, those scientists who agree with referring to the concept by the name atomic weight point out, among other things, that it is the name it has received continuously since 1808 , when the English chemist John Dalton conceptualized it by first time. Two centuries are too long to eradicate its use based simply on semantic issues, since the imprecision of the name can be accepted and not allow that to generate confusion regarding what it represents.
Points in favor of the notion
Other points that support the use of atomic weight are the following:
* Although for a long time atomic weight was measured through gravimetry (also called gravimetric analysis ), a method that involves the action of weighing, the fact that this has changed does not justify the modification of the name originally assigned to the concept of this physical quantity ;
* the name “relative atomic mass” should be reserved for the mass of a specific isotope (or nuclide);
* There are various examples of names of physical quantities that do not adequately represent their meaning, such as resolving power , molar concentration and electromotive force , which are not force or power or molar quantity, respectively.
Atomic weight… which is not atomic
Finally, being truly meticulous one can argue that atomic weight is not really "atomic", since it does not refer to a single atom. Of course, the same can be argued against the use of "relative atomic mass" when referring to the same concept.
This phenomenon of misnamed names also occurs outside of science and, similarly, creates divisions in academia. However, the name chosen in no way reflects the skill of a scientist, but rather a personal preference that may respond, for example, to a need to organize concepts in one's mind in a certain way.
