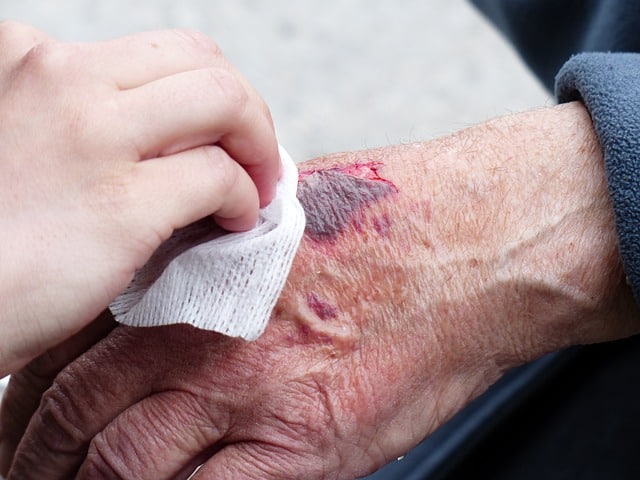
Hydrogen peroxide is used to clean wounds.
Peroxide is a notion used in chemistry to name the oxide that has the highest number of oxygen among all possible ones. It can be said, therefore, that a peroxide is an oxide that has a higher level of oxygen than ordinary oxides.
The structure of a peroxide is given by a covalent bond between one oxygen and another oxygen . Oxygen, in a peroxide, has an oxidation state equivalent to -1 .
Characteristics of a peroxide
Peroxides have the ability to cause explosions or fire when they come into contact with a flammable substance. Due to this particularity, working in a laboratory with this type of elements must be very careful, since accidents can be potentially lethal.
With peroxides, it is possible to create products with the ability to disinfect, fuels, dyes and many other substances that are very useful at an industrial or domestic level, depending on the case.
The hydrogen peroxide
The most common peroxide is hydrogen peroxide , popularly called hydrogen peroxide when it is present in a certain type of aqueous solution. This liquid, which can also generate a solution with alcohol, has multiple uses.
Hydrogen peroxide, for example, can be used to clean wounds since, thanks to its oxidizing action, it helps fight bacteria or microbes. Depending on the degree of dissolution, hydrogen peroxide can also be used to bleach fabrics or papers, to produce certain oils or as a reagent.
Due to its characteristics, hydrogen peroxide helps fight microbes.
Risks when using hydrogen peroxide
It is important to know that hydrogen peroxide can be harmful to health in certain cases. Thus, it is established that it can have negative effects in different situations and based on certain statements:
-People who access solutions where this element is present in a higher concentration than recommended may suffer problems such as eye irritation and allergic skin reactions. All this without forgetting strong irritations in other parts of the body, such as the stomach, intestines or even the inside of the mouth.
-No less important is to know that if someone ingests a large amount of hydrogen peroxide they will die.
-Although there are no studies that specifically determine this, we cannot forget that experts in the field have indicated that this peroxide has the capacity to stimulate both the growth and multiplication of the cells that cause cancer.
Teeth whitening
We must not forget that hydrogen peroxide is used when whitening teeth. And it offers great results in terms of removing stains that may have appeared on the teeth. Specifically, it must be stated that it can be used in several different ways with the same objective: through gels that are applied directly to the teeth, through strips that are glued to them or thanks to covers that contain it and that are made tailored to each person.
Other common peroxides are benzoyl peroxide , sodium peroxide , manganese peroxide , and acetone peroxide .
