 Paramagnetism is called a type of magnetism in which a material is weakly attracted by a magnetic field that is activated from the outside . This means that what is paramagnetic has a certain susceptibility to a magnetic field .
Paramagnetism is called a type of magnetism in which a material is weakly attracted by a magnetic field that is activated from the outside . This means that what is paramagnetic has a certain susceptibility to a magnetic field .
When the external magnetic field is removed, the paramagnetic material fails to maintain magnetic properties . The conditions of paramagnetism are linked to the realignment that the external magnetic field generates in the electron trajectories and to the fact that the material has unpaired electrons.
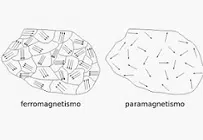
It can be stated that paramagnetism reflects the tendency of free magnetic moments to be arranged parallel to a magnetic field . When magnetic moments establish a strong bond with each other, this is ferromagnetism .
The absence of an external magnetic field means that the magnetic moments do not align in parallel, but rather arrange themselves randomly . The influence of the external magnetic field, on the other hand, causes the tendency of parallel alignment.
A ferromagnetic material, in short, reflects a weak attraction by an external magnetic field . The attraction is not enough to produce the phenomenon known as ferromagnetism. Paramagnetism, furthermore, involves the fading of magnetic properties when the magnetic field acting from the outside disappears: the material in question is therefore not permanently magnetized .
Aluminum , magnesium , lithium , titanium , and platinum are some of the materials with paramagnetism properties. This quality can be used in different ways at an industrial level.
In this framework we must talk about Curie's law , whose name refers to its creator, the French scientist Pierre Curie , one of the pioneers in the field of radioactivity. In its definition it is stated that when the magnetic fields are low, we can appreciate a magnetization in the paramagnetic materials that is found in the reaction of the external field and its magnitude is described using the following equation : M = χH = C/TH .
Let's see below the meaning of each of the variables in the equation just presented:
 * χ, the twenty-second letter of the Greek alphabet, pronounced ji in our language, in this case is used to refer to molar magnetic susceptibility . This value represents the magnitude of a given substance's susceptibility to magnetization. If it is positive, the magnetization acts by reinforcing the field, while if it is negative it opposes it;
* χ, the twenty-second letter of the Greek alphabet, pronounced ji in our language, in this case is used to refer to molar magnetic susceptibility . This value represents the magnitude of a given substance's susceptibility to magnetization. If it is positive, the magnetization acts by reinforcing the field, while if it is negative it opposes it;
* M is the result of the equation, that is, the magnetization value obtained after having resolved all the other unknowns;
* H is the strength of the magnetic field , the physical magnitude that describes its vector. Depending on the source, some refer to this concept by the name magnetic flux density , while others call it vector magnetic induction ;
* T denotes the absolute temperature measured in kelvin . Formerly, this unit was called the degree Kelvin and was created in the mid-19th century by William Thomson , Baron Kelvin;
* C indicates the material constant, which is specific to each one. In more technical terms, this variable is known as the Curie constant .
Curie's law tells us that paramagnetic materials have a tendency to increase their magnetism as the applied field increases, and to reduce it if the temperature is raised. It is important to note that it can only be applied at high temperatures or low fields, because if almost all the magnetic moments are aligned it does not provide correct results. In a case like this, close to magnetic saturation, the response is no longer linear and the magnetization stops growing.
