 The mass number is calculated by adding all the neutrons and protons present in the nucleus of an atom . The same element can have different mass numbers according to its isotopes .
The mass number is calculated by adding all the neutrons and protons present in the nucleus of an atom . The same element can have different mass numbers according to its isotopes .
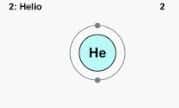
It should be remembered that an atom is a particle that cannot be divided by chemical methods. Atoms have a nucleus made up of protons (which have a positive electrical charge) and neutrons (which have no electrical charge).
The atom is precisely the smallest constituent unit of matter, within which it retains the characteristic properties of a chemical element. All liquids, solids, plasmas and gases have atoms in their composition, whether neutral or ionized. The size of an atom makes it impossible to see without the help of a microscope, since it is around 100 picometers (one meter divided into ten billion).
We also mention the concept of a chemical element , a class of matter that is made up of atoms, all of them belonging to the same type. If we take as an example the simplest possible chemical element, we can affirm that in its nucleus it has a defined number of protons and that it belongs to a single category that is classified according to its atomic number, even when there are several atomic masses for it.
Going deeper into the definition of a proton, it is a subatomic particle, since it is smaller than the atom, and its electric charge has the same value as that of the electron, but with the opposite sign, in addition to exceeding its mass 1836 times. It is considered stable although some scientists claim that it could disintegrate and give rise to other particles . The first observations of the proton gave rise to the idea that it was an elementary particle, one that does not have smaller ones in its constitution; However, since the 1970s there has been evidence to the contrary.
Both the proton and the neutron can be called nucleons . It is necessary to note that not all atoms contain neutrons; protium is an exception. Although it is stated that the neutron has no electrical charge, it is made up of three fundamental particles ( quarks ) that are charged, although the sum of their charges is zero.
To know the mass number of a chemical element, you must add the neutrons and protons in its nucleus. In the case of isotopes , these are the various elements that share the number of protons, but have different quantities of neutrons. Thus, isotopes of an element have different mass numbers.
 Almost all chemical elements have more than one isotope, except for eight of them, including sodium and beryllium. Tin, for its part, has the most: 10.
Almost all chemical elements have more than one isotope, except for eight of them, including sodium and beryllium. Tin, for its part, has the most: 10.
It is important not to confuse the mass number with the atomic number , which refers specifically to the number of protons , without considering neutrons. For this reason, the mass number is usually higher than the atomic number.
This link allows us to state that the mass number is equal to the sum of the atomic number (that is, the number of protons) and the number of neutrons . Similarly, the number of neutrons in an atom can be calculated by subtracting the atomic number from the mass number:
Mass number = Atomic number + number of neutrons
Number of neutrons = Mass number – atomic number
To indicate the mass number, a superscript is used that is located to the left of the chemical element symbol, above the atomic number. Suppose that an iron atom ( Fe ) has 26 protons and 24 neutrons . Its mass number, in this case, is 50 ( 26 protons + 24 neutrons ) and is written as a superscript on the left side of Fe and above 26 (the atomic number).
