The atomic number reflects how many protons there are in the nucleus of an atom.
Atom is the smallest portion of any chemical element, which cannot be divided and has independent existence. Atoms are made up of electrons orbiting a nucleus with neutrons and protons .
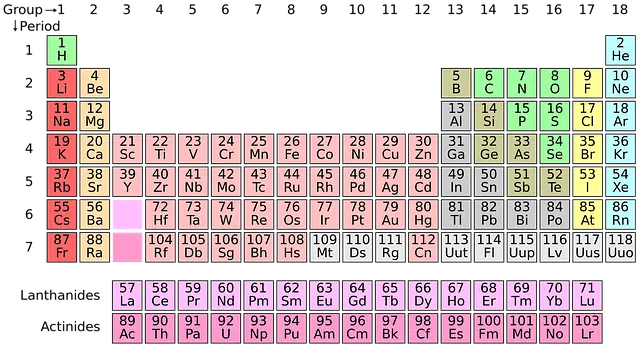
Atoms of different elements have different numbers of protons . The atomic number (identified with the letter Z , from the German term zahl ) indicates the number of protons present in the nucleus of an atom. This number , therefore, is responsible for defining the electronic configuration of the atom and allows the ordering of the various chemical elements in the periodic table , which begins with hydrogen (Z=1) and continues with helium, lithium, beryllium, boron, carbon and nitrogen.
We also have to add that the number of protons that exists in the nucleus of a specific atom is equal to the number of electrons that surround it in what is called the crust.
Chemical elements according to atomic number
The list of chemical elements established based on their atomic number can be determined to be headed by these ten elements: 1 is hydrogen, 2 is helium, 3 is lithium , 4 is beryllium, 5 is occupied by boron, 6 is carbon, 7 is nitrogen, 8 is oxygen, 9 is fluorine and 10 is neon.
To this we can also add that in total this list is made up of a total of 115 chemical elements, the last of them being ununoctium. Presumably this is a colorless noble gas that has the following temporary symbol: Uuo.
It is important to keep in mind that an unaltered atom is electrically neutral : this means that its atomic number will always be the same as its number of electrons.
The atomic number defines the electronic configuration of the atom.
The work of Meyer, Mendeleev and other scientists
Although the periodic table of the elements is usually attributed to Dimitri Mendeleev (responsible for arranging the elements according to variations in chemical properties), the person who specified the organization according to the physical properties of the atoms was Julius Lothar Meyer .
However, we cannot ignore the important role played by the English physicist and chemist Henry Moseley who established his own law of atomic numbers, called Moseley's Law. In 1913 it was announced that it established that there was a clear systematic relationship between the wavelength of the X-rays that were emitted by atoms and their atomic number.
In this way, with this discovery, this British chemist came to oppose or counteract the proposals that the aforementioned Mendeleev carried out four decades earlier.
Relationship between atomic number and mass number
We have already said that the number of protons (expressed by the atomic number) is equal to the number of electrons. The mass number ( A ), for its part, indicates the number of particles that an atom has in its nucleus. Therefore, it expresses the sum of protons and neutrons.
It is possible to establish, in short, the following relationship between the atomic number and the mass number: A=Z+N , where N is equivalent to the number of neutrons.
