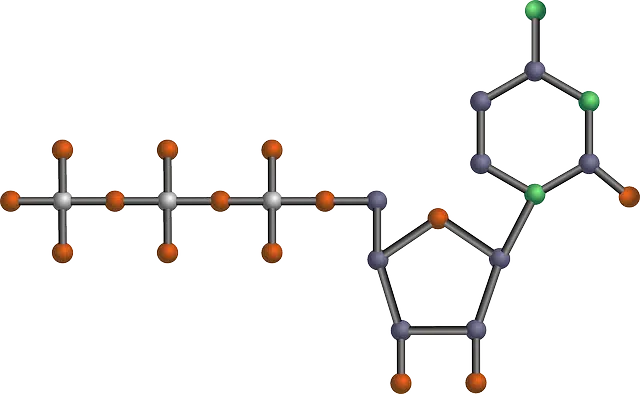
Nucleotides are made up of a nitrogenous base, a sugar and phosphoric acid.
A nucleotide is an organic compound that is made up of a nitrogenous base , a sugar and phosphoric acid . It is possible to divide nucleotides into ribonucleotides (when the sugar is ribose) and deoxyribonucleotides (if the sugar is deoxyribose).
Nucleotides can act as monomers in nucleic acids (DNA or RNA), forming linear chains, or act as free molecules (as is the case of ATP).
The nitrogenous base of the nucleotide can be purine (adenine or guanine), pyrimidine (thymine, cytosine or uracil) or isoalloxacin (flavin). DNA is formed with adenine, guanine, thymine and cytosine, while RNA involves adenine , guanine, cytosine and uracil.
Nucleotide according to its base
Nucleotides with a purine or puric base are called adenosine (for the adenine base) or guanosine (guanine base). Instead, pyrimidine base nucleotides are known as thymidine (thymine base), cytidine (cytosine base) or uridine (uracil base).
The nucleotide sugar, for its part, belongs to the pentose group since it has five carbon atoms . It can be ribose or deoxyribose.
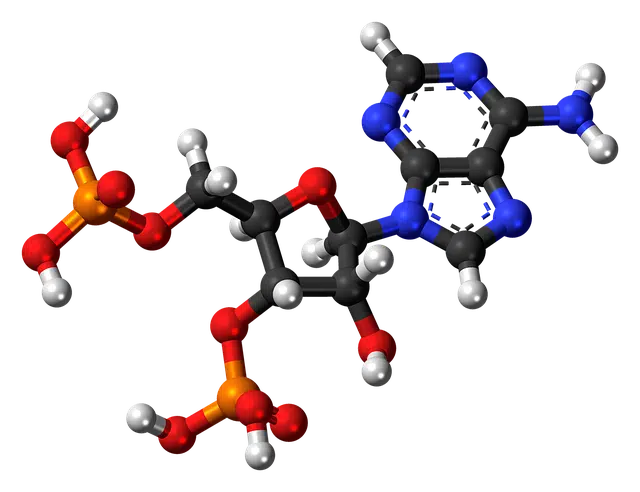
There are different types of nucleotides.
phosphoric acid
Regarding phosphoric acid, each nucleotide can contain one ( nucleotide-monophosphate ), two ( nucleotide-diphosphate ) or three ( nucleotide-triphosphate ). These phosphate groups give the nucleotide a high- energy bond, so they are taken as sources for energy transfer by cells .
When the nucleotide has only one phosphate group, it is correct to say that it is stable. Instead, with each additional phosphate group, the nucleotide becomes more unstable and the phosphorus-phosphate bond releases energy by breaking through hydrolysis .
Non-nucleic nucleotides
Non-nucleic nucleotides are very important for biology , as well as those that are part of nucleic acids.
They live freely in cells and participate in their metabolism and, as enzyme activators, in their regulation, providing them with chemical energy during their reactions. One of them, the ATP, is mentioned in previous paragraphs.
ATP and ADP
ATP and ADP are important nucleotides for biology, since the bonds that form the phosphate groups are very rich in energy (in fact they are molecules that are dedicated to the transport of energy), which accumulates at the time of their union and It is easily released when the bond is broken by hydrolysis.
In addition to being one of the two most important energy transporters, ATP is a very versatile element in various energy exchanges: starting from phosphoric acid and ADP (phosphorylation), ATP is formed with the energy released during exergonic reactions (characterized due to presenting a negative variation of the Gibbs free energy); When dephosphorylation occurs, that is, ATP is hydrolyzed (interacts with water and modifies its structure) to phosphoric acid and ADP, the energy released from this process serves to fuel the endergonic reactions (the Gibbs free energy presents a variation positive).
Coenzyme nucleotides
Coenzymes are non-protein organic molecules that participate in enzymatically catalyzed reactions, during which they are usually responsible for the transport of electrons. A large number of these coenzymes are nucleotides, although they also exist in other classes. Unlike enzymes, they do not depend on a particular type of substrate to act in a given reaction.
Flavin nucleotides, for example, which are FMN (flavin-mononucleotide, in which there is a bond between a phosphate group and the flavin) and FAD (flavin-adenine-dinucleotide, which is formed by a molecule of FMN linked through a phosphodiester bond to one of AMP), are coenzymes, and can be found in oxidized form (with the names just mentioned) or reduced (under the names FMNH2 and FADH2).
