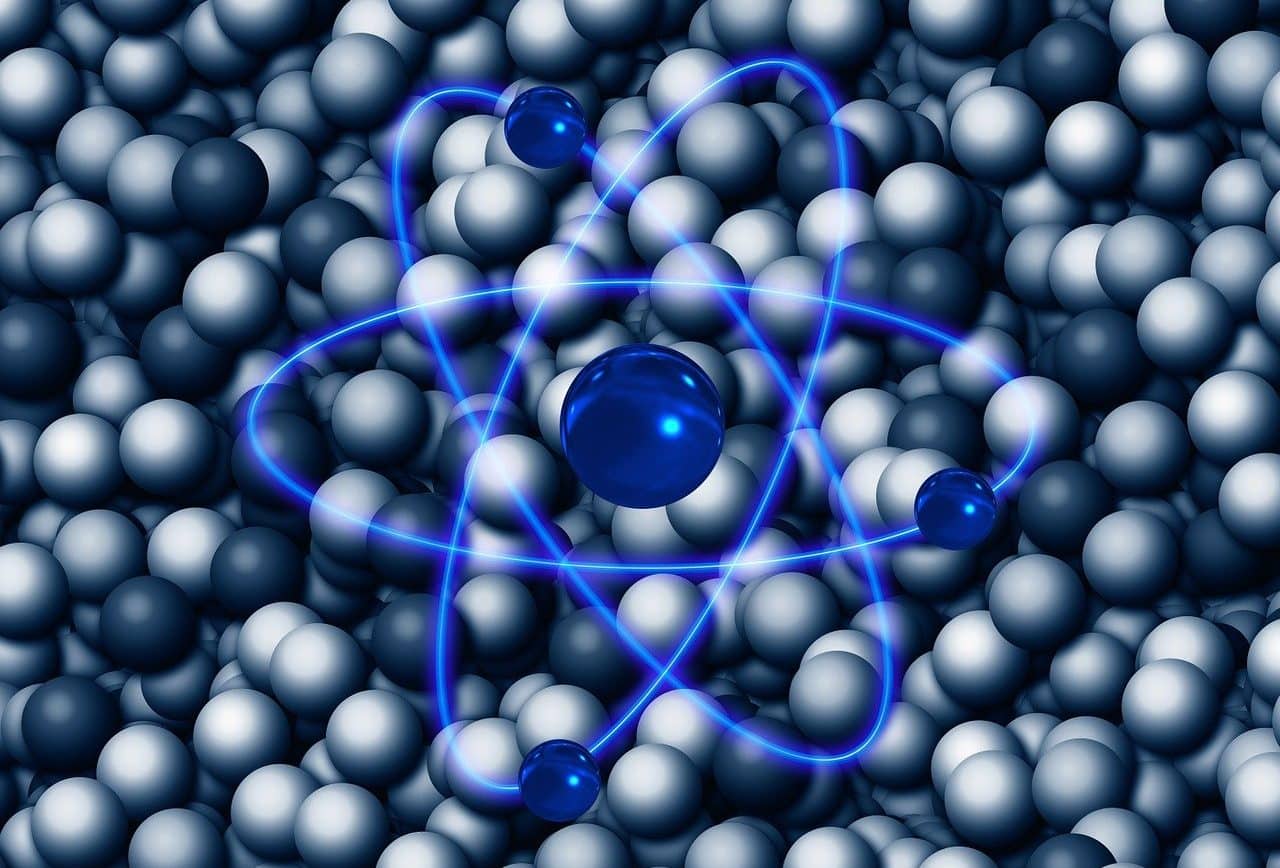
After absorbing energy, an electron has the possibility of making a jump that takes it from a fundamental state to another of the quantum states with more energy, classified as excited.
Energy levels come into play and become visible in multiple contexts. In the field of health, for example, this idea is related to the strength or vitality that can be gained or lost depending on someone's age, lifestyle or habits. Not having a balanced and healthy diet, not getting enough rest and illnesses, for example, lead to loss of energy, just as doing physical activity , eating properly and sleeping well contribute to raising energy levels .
In quantum mechanics , chemistry and in the branches of both so-called particle physics and nuclear physics this expression is also present, although associated with issues such as atomic orbitals , quantum numbers , the Schrödinger equation , electronic configuration and Bohr atomic model (which encompasses a postulate that states that electrons emit or absorb energy only when jumping from one allowed orbit to another, generating or capturing a photon whose energy value arises from the energy difference between the different levels involved), For example.
Learning what this notion is about and what particularities and scope it has according to its field of application allows us to understand and explain numerous phenomena and realities. By focusing on atoms, specifically, it is possible to appreciate and understand the importance that energy levels achieve since they significantly influence the way atoms interact.
The theory states that, with the atomic absorption of energy , each electron moves to a higher energy level , while the energy loss causes the electrons to descend in level. Experts on the subject indicate that electronic transitions stimulate the generation or impregnation of electromagnetic radiation manifested in photons , establishing the basis of spectroscopy in this framework.
By looking at the periodic table it is possible to find a wide range of energy levels and sublevels in which electrons are positioned. It is useful, at the same time, to master the resources of quantum physics in order to take advantage of the wave-mechanical model linked to atoms to predict main energy levels .
Rules related to energy levels
When the knowledge regarding electronic configuration is deepened, rules and exceptions related to energy levels are revealed.
One of these postulates is the so-called Pauli exclusion principle , which is key to understanding several characteristic features of matter (such as its volume and stability). They are a consequence of it, for example, some magnetic, mechanical, chemical, optimal and electrical properties of solids.
There have long been researchers focused on investigating non-reciprocal electrical transport, chiral superconductivity and how to make superconducting diodes that could be used in superconducting circuits.
The octet rule or theory , content that states that, regardless of the variety of chemical bond studied, the outermost level will always be surrounded by eight electrons to achieve stability , except in specific cases. Atoms belonging to noble gases , to mention a common reference, reflect an electronic structure with complete levels and sublevels based on an octet of electrons .
An interesting approach to the explanation of conductivity in metals is achieved by applying the Fermi-Dirac principle to conduction electrons (whose distribution is essential to analyze free electrons in metals).
Practical applications
In practice, energy levels have a wide range of applications. They influence, as can be seen from reality, the operation of lasers , as the name of an amazing quantum process and devices where energy is emitted as electromagnetic radiation .
Nor can we overlook the great discovery of three researchers regarding the synthesis and use of quantum dots , fundamental elements to give impetus to nanotechnology . As a reward for their scientific contributions in this field, Alexei Ekimov , Moungi Bawendi and Louis Brus were awarded the 2023 edition of the Nobel Prize in Chemistry . In everyday life, quantum dots (whose optoelectronic properties give the possibility of, with their color or wavelength, tuning each energy level ) serve for professionals linked to the health field to map biological tissues, to give them nuances lighting to LED lamps and to illuminate television and computer screens.
The creation of solar energy with quantum characteristics capable of self-regulation at a thermal level and quantum dots at the service of more efficient solar cells are two of the challenges that many scientists face.
Challenges in manipulating and interpreting energy levels
Although much progress has been made and scientific research is ongoing, there are still challenges related to the manipulation and interpretation of energy levels .
In this framework there is attractive technological potential , improvements to be implemented and hundreds of questions that will surely find answers thanks to the work of researchers.
Some time ago, for example, the exact location of the internal point of an atom where quantum light is born was announced: this benefits, thanks to the fact that interferometers can be optimized, astrophysics, medical explorations and quantum computing, among other areas.
