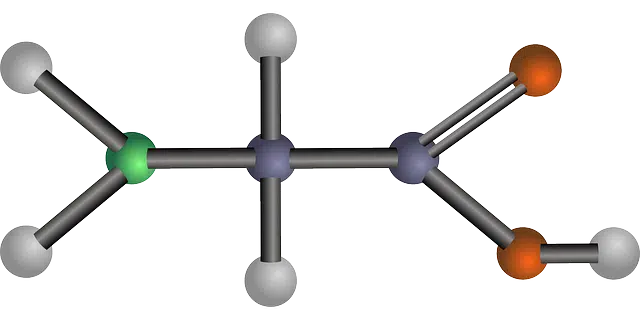
Monomers are molecules that have a reduced molecular mass.
Monomers are molecules that have a reduced molecular mass . When many monomers are joined together through chemical bonds, they form a polymer , which is a macromolecule .
Molecular mass is a quantity that refers to the number of times the mass of the molecule is greater than the atomic mass unit. Its value is coincident with that of the molar mass or molecular weight , although it is expressed in different units. If the molecular mass is small, the molecule is called a monomer .
Polymerization
The process of combining monomers to form a polymer is called polymerization . Two monomers joined together form a dimer , while three monomers give rise to a trimer . The polymer developed with more than three, although with a limited amount of monomers, is known as an oligomer .
Polymers, in short, are chains of monomers - generally linked by covalent bonds - that have various molecular weights . Natural monomers , of natural origin as their name indicates, allow the conformation of biomolecules found in living beings.
Synthetic monomers , on the other hand, are used to make plastics and paints. These are artificial monomers such as tetrafluoroethylene and acrylamide , among many others.
Through polymerization, many monomers can come together and form a polymer.
Different types of monomers
Proteins , for example, are macromolecules that are created from the bond established by different amino acids . Among these monomers we can mention cysteine , glutamine , leucine and tryptophan . Monosaccharides , meanwhile, are the monomers of carbohydrates or carbohydrates .
In addition to amino acids, there are other types of monomers, such as nucleotides , organic molecules that are formed when a five-carbon monosaccharide (called pentose ), a phosphate group and a nitrogenous base are covalently linked. A covalent bond occurs between two atoms when they join together and share electrons.
The monosaccharides
Monosaccharides are also known as simple sugars and are the least complex carbohydrates, those that are not decomposed into others of less complexity, that is, they are not hydrolyzed. A phosphate group is composed of a polyatomic ion and a molecular mass. A nitrogenous base is an organic compound in which there are a minimum of two nitrogen atoms.
Although nucleotides include monosaccharides in their composition, the latter are also monomers. The most important of this group is glucose , which is the most common cellular energy source. Its carbon chain (that is, its basic structure, formed by carbon atoms to whose covalent union other oxygen, nitrogen or hydrogen atoms are also attached) is not branched and only one of its carbon atoms does not contain an alcohol group.
Another way to define monosaccharides is as the monomers of so-called polysaccharides , which are also known as carbohydrates or simply sugars .
Isoprene is also a monomer. It is an organic compound that at room temperature is a colorless liquid with great volatility. The latter is because its boiling point is very low. Likewise, it is easy to ignite and very flammable. Its reactivity is especially high when it comes into contact with air, and it can explosively polymerize if its temperature increases considerably.
