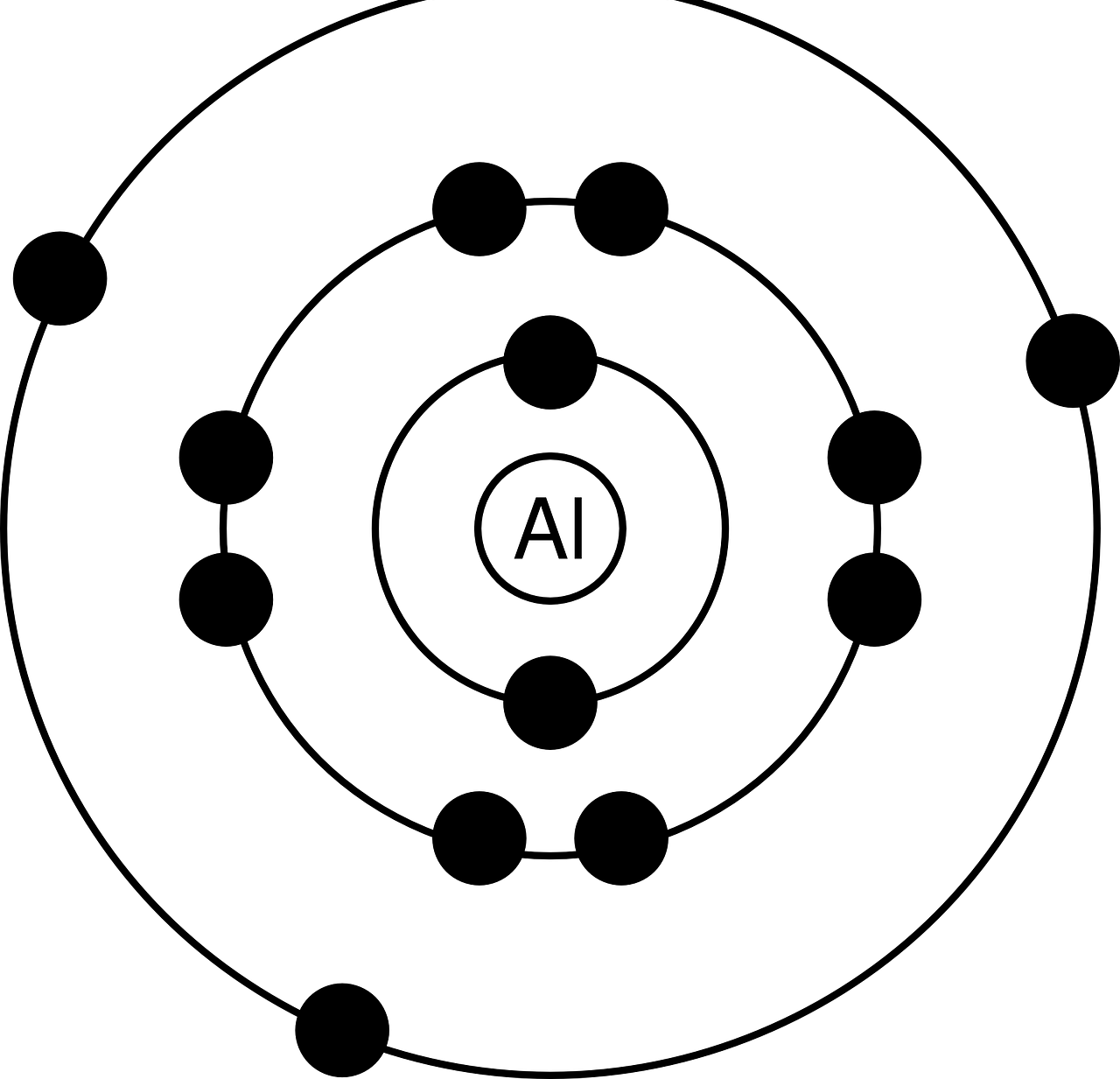
Niels Bohr launched an atomic model (identified with his last name by way of recognition) in which he realizes the existence of electrons occupying stable orbits around an atomic nucleus.
The Bohr atomic model is a presentation focused on atoms made in 1913 by a prominent Danish physicist named Niels Bohr . This contribution was motivated by the intention, taking Rutherford's atomic model as a starting point, to provide an explanation in relation to how matter achieves stability . Bohr also dealt with quantization and tried to provide answers linked to both the emission spectrum and the absorption spectrum observed when analyzing gases.
The result was a proposal that alludes to the stable orbit that, around the atomic nucleus , an electron can have, in addition to not overlooking the particular emission spectra presented by atoms . When enriching and strengthening the content of this model , issues associated with the photoelectric effect that scientists such as Heinrich Hertz and Albert Einstein , among others, knew how to study and describe so well, were taken into account.
When drawing up his scheme, Bohr proposed that electrons (as subatomic particles with a negative electrical charge are called) are arranged, in a concentric style, in circular orbits surrounding the nucleus .
Features of the Bohr atomic model
Bohr's atomic model brings together characteristics that arose from ideas or thoughts that Niels had when he wanted to clarify doubts or improve the atomic model that had previously come to light at the hands of Ernest Rutherford (a physicist and chemist who, in turn, of Thomson's atomic model and reached relevant findings and results when carrying out the so-called gold foil experiment ).
In principle, he made a description of an atom corresponding to hydrogen in whose nucleus there was a proton , as well as an electron rotating around it. The expert interpreted, in this context, that electrons could only move in certain orbits depending on the energy levels . This is how we arrived at the assignment of a figure that in the field of quantum mechanics is defined as the principal quantum number . Based on this, and suspecting that each of the electrons possessed a quantized angular momentum that could only be modified in whole fractions associated with Planck's constant , Bohr calculated the corresponding distances between the nucleus and the different orbits present in the atom. of hydrogen .
The Bohr model , unable to fully adapt to atoms of elements other than hydrogen in terms of spectra , was perfected in 1916 by Arnold Sommerfeld (who appealed to concepts from the theory of relativity to introduce changes with respect to the Bohr model ). .

The photoelectric effect explained by Albert Einstein was considered in the development of the Bohr atomic model. It should be noted that both scientists debated and participated in disputes in relation to quantum mechanics.
It is also necessary to point out that there were three essential postulates that led to the atomic model promoted by Bohr . The first states that electrons do not radiate energy when describing a circular orbit around the atomic nucleus . Secondly, it is indicated that only orbits with an angular momentum that is an integer multiple of Planck's constant are allowed for an electron . Finally, progress was made on a premise aimed at the ability of electrons to emit or absorb energy only when making the jump from one orbit to another. In this process, a photon is generated or attracted, which exhibits an energy that arises as a difference in energy between the levels involved.
Importance
Bohr's atomic model is important because, in addition to allowing deeper knowledge about the atom , it served to better understand electrons .
Although it was necessary to continue research to respond to structures more complex than that of hydrogen , Niels Bohr undoubtedly opened a path of learning that was enriched with the passage of time and laid the foundations for the atomic model that governs today. . It also gave support to the essence of quantum mechanics .
Of course, these contributions have had limitations (they did not provide an explanation related to stable orbits at the time, for example). Fortunately, scientists appeared who improved Niels ' legacy with findings, conjectures and experiments. This was the case with Louis de Broglie , responsible for the idea of the existence of wave-particle duality in electrons . This hypothesis was confirmed in 1927 when Lester Halbert Germer and Clinton Joseph Davisson carried out a test called the Davisson-Germer experiment .
Each distance at which electrons are located depending on their location with respect to the nucleus of an atom is known as an energy level.
It cannot be overlooked that Niels ' atomic approach has never lost relevance: proof of this is that, to indicate another case as a reference, it served Erwin Schrödinger , who also used wave-particle duality , to the discovery of the so-called Schrödinger equation . It is a non-relativistic postulate identified as a vector equation within which particle-antiparticle annihilation or the creation of pairs are not contemplated.
