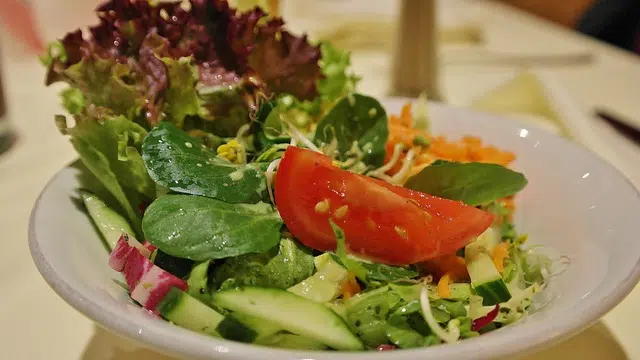
In a heterogeneous mixture, the components can be easily distinguished.
The addition or addition of different elements or substances that lack a chemical interaction with each other is called a mixture . In a broad sense, a mixture arises when joining or combining various bodies.
Heterogeneous , meanwhile, is an adjective that comes from the Low Latin heterogeneus , in turn derived from the Greek heterogenḗs . The concept refers to that which is formed with components of a different nature.
What is a heterogeneous mixture
A mixture whose composition is not uniform is called a heterogeneous mixture , making it possible to differentiate its components easily. These mixtures are developed with at least two substances that are dissimilar from a physical point of view and that are distributed unevenly in the combination.
Broadly speaking, the parts that make up a heterogeneous mixture can be separated or isolated without major complications . Depending on the characteristics of the components, these heterogeneous mixtures can be considered suspensions or coarse mixtures .
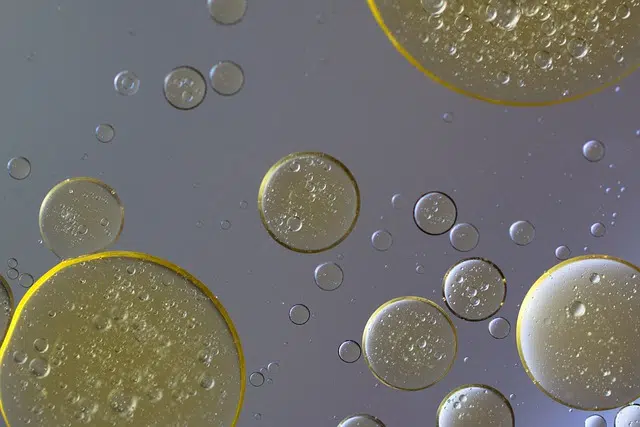
Water and oil make up a heterogeneous mixture.
Lack of homogeneity
It is easy to understand what a heterogeneous mixture is if a comparison is made with homogeneous mixtures . In the case of a homogeneous mixture, it includes uniform components; thus, they cannot be easily distinguished.
Homogeneous mixtures are solutions (also known as dissolutions ). They are formed with a solvent (that which dissolves) and a solute (the part that dissolves).
Suppose sugar is added to water : when the sugar dissolves, a homogeneous mixture is obtained. On the other hand, if stones are placed in the water, a heterogeneous mixture will be produced.
Main characteristics of a heterogeneous mixture
As a summary, it can be indicated that a heterogeneous mixture is characterized by presenting a composition that lacks uniformity. Due to this particularity, its components can be distinguished with the naked eye.
By extension, the parts of the heterogeneous mixture can be separated easily. Depending on the situation, it is possible to resort to physical procedures that serve to isolate each phase .
Continuing with the analysis of heterogeneous mixtures, it should be noted that this dispersion between the particles leads to no chemical reactions being recorded. There is no complete integration or fusion of the ingredients
Some examples
Coarse heterogeneous mixtures are easily recognizable. A tomato and lettuce salad is an example : you can clearly see that there are two types of elements (tomato and lettuce), which can be taken separately.
If we place stones inside a container with sand, we also get a heterogeneous mixture. The same thing happens if, in a bowl, we mix nuts .
Water and oil , likewise, create a heterogeneous mixture. Due to their different densities, there is no uniformity, just as there is with water and gasoline.
To separate the substances that are part of a heterogeneous mixture, different techniques can be applied. Filtration , sieving and decanting are some of the most commonly used procedures. The choice of one or the other, of course, must respond to the characteristics of the mixture: trying to separate the ingredients of a salad is not the same as isolating water from gasoline, to mention a few possibilities.
