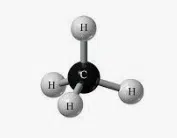
 Methane is a hydrocarbon that is made up of four hydrogen atoms and one carbon atom . With the chemical formula CH4 , under normal conditions of temperature and pressure it appears as a gas.
Methane is a hydrocarbon that is made up of four hydrogen atoms and one carbon atom . With the chemical formula CH4 , under normal conditions of temperature and pressure it appears as a gas.
This element is formed when organic matter decomposes. It is also generated in coal mines. It is an odorless and colorless substance in which hydrogen atoms bond to carbon through a covalent bond.
Digestion and defecation of livestock; the bacteria present in rice plantations; the extraction of fossil fuels; and swamps are other sources of methane.
It should be noted that methane is the main component of natural gas . This hydrocarbon is obtained from the mixture of various light gases that have a natural origin. Sometimes helium , nitrogen, carbon dioxide and other elements are added.
Used as fuel in steam generators and turbines, methane is used by humans to generate electricity . It is also used as a raw material to obtain methanol, hydrogen and other chemical products.
It is important to mention that methane is part of the so-called greenhouse gases. These are atmospheric gases responsible for the absorption and emission of radiation in the infrared range; The process called the greenhouse effect consists of the permanence of part of that radiation in the soil, since the gases return it, and in this way increase its temperature above natural values.
The presence of methane in the Earth's atmosphere has grown in the last 5,000 years, contributing to global warming . It has even been detected in the atmosphere of Mars . This discovery, made in 2003 , leaves open the possibility that life exists on said planet.
In more precise data, we can say that in a period of one hundred years, for each kilogram of methane the planet will warm up to twenty-three times more than with the same amount of carbon dioxide . To look at the positive side, we must not overlook that in the Earth's atmosphere the proportion of carbon dioxide is much greater than that of methane, so the latter does not contribute as much to the greenhouse effect.
 Although it lacks toxicity , methane can become dangerous because it is very flammable : its ignition can therefore cause burns. For it to burn, there must be an ignition source and it must be mixed with air in concentrations ranging from 4.5 to 15 percent.
Although it lacks toxicity , methane can become dangerous because it is very flammable : its ignition can therefore cause burns. For it to burn, there must be an ignition source and it must be mixed with air in concentrations ranging from 4.5 to 15 percent.
The fact that it is not toxic does not make it a healthy product either, but rather gives us a certain margin of exposure to it without our body being harmed. For example, if by accident or due to the characteristics of our workspace we inhale it in small quantities , there is no risk of harm. On the other hand, if we are in a closed space and the volume of methane is considerable, it can cause asphyxiation, since, like natural gas, it is capable of displacing air.
Many natural gas supply companies offer safety programs to avoid these risks. For example, it is common for them to add an easy-to-distinguish odor to the gas so that their customers can detect leaks and contact the emergency team immediately.
The most important reactions of methane are steam reforming to obtain synthesis gas, halogenation and combustion. In general, it is not easy to control these reactions, something that can be seen in particular with the partial oxidation to obtain methanol, which usually passes to water and carbon dioxide.
