
The periodic law states that the physical and chemical properties of elements tend to repeat themselves systematically as the atomic number increases.
The periodic law is the basis of the periodic table of elements . This law states that the chemical and physical properties of elements tend to repeat themselves systematically as the atomic number increases. The table, therefore, is a kind of scheme that is responsible for arranging the chemical elements according to the increasing order of atomic numbers.
A British chemist named John Alexander Queen Newlands ( 1838 – 1898 ) was one of the precursors of this concept by proposing the law of octaves , which indicated that every eight elements found similar properties. Under this idea, Newlands created a periodic table in 1863 .
Meyer's contribution
The German chemist Julius Lothar Meyer ( 1830 – 1895 ) relied on these notions to discover the atomic volumes of the elements.
After calculating the different atomic weights and making graphs with these values, this expert managed to demonstrate that the increase in atomic weight corresponded to an increase in physical properties. Meyer 's works regarding the periodic law were published in 1870 .
Mendeleev's table and the periodic law
The Russian chemist Dmitri Mendeleev ( 1834 – 1907 ), however, is the one who has the historical merit as creator of the periodic table of elements. His work consisted of arranging the elements according to their atomic mass and placing those that had something in common in the same column. His table, presented in 1869 , was based on manual alteration of chemical properties.
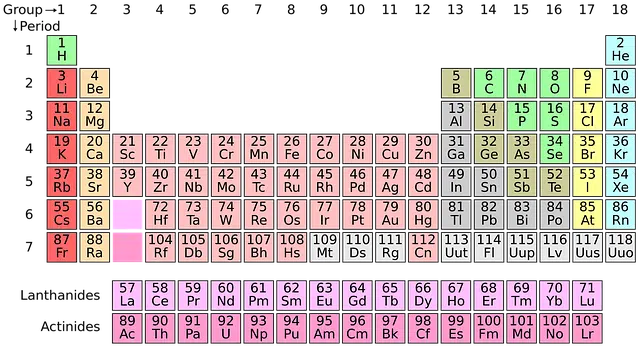
The vertical columns of the periodic table are known as groups and include elements with identical atomic valence (and, therefore, have similar properties to each other). The horizontal rows, for their part, are called periods and group elements with properties that are different but whose masses are similar.
Dmitri Mendeleev relied on the periodic law to create his table of elements.
Moseley's work
This empirical law , authored by British physicist Henry Moseley in 1913, defines that when an atom emits an X-ray there is a systematic relationship between its atomic number and wavelength.
The importance of this law lies in the fact that it left behind the notion that the atomic number was simply the representation of the position that each element had in the periodic table, which had been assigned almost without specific reason by Mendeleev.
From then on, Moseley undertook a number of experiments to confirm the Bohr model of the atom (also proposed in 1913 by the Danish physicist named Niels and which states that electrons can orbit stably around the atomic nucleus , among other things. of great importance) in X-ray energies, starting from the measurement of the frequencies that originate in the electronic transitions that heavy atoms undergo.
The table in the form of a periodic table
While the periodic table represents a nightmare for many high school students, it generates a fascination for others beyond belief. This is the case of Theodore Gray, co-founder of Wolfram Research, one of the most respected software and scientific and technical innovation companies, who in 2002 built a table in the shape of a periodic table, which contains samples of each element located according to Dmitri Mendeleev's distribution.
His work did not end there, but he began to distribute different designs of his creation to museums and schools, placing special emphasis on the importance of appreciating the elements in person, as opposed to simply seeing their nomenclature on paper. Although this man does not have the fame of a popular singer, his active collaboration with science is well recognized among people who like this branch of knowledge, and there is a lot of information about him and his discoveries on the Internet.
