Electroplating allows a metallic layer to be applied through an electrolysis process.
The etymology of electroplating takes us to the French word galvanoplastie . The formation of the concept refers to Luigi Galvani , an Italian physicist, physiologist and doctor born on September 9, 1737 in Bologna and died on December 4, 1798 .
Galvani made important discoveries linked to the electrical properties of nerve impulses . For the development of the term galvanoplasty, his surname was associated with the compositional element -plastia , which refers to a "reconstruction" .
What is electroplating
Electroplating is the application of a layer of metal on a body through a process of electrolysis . The idea of electrolysis or electrolysis , meanwhile, refers to breaking down a substance that is in solution into ions using an electric current .
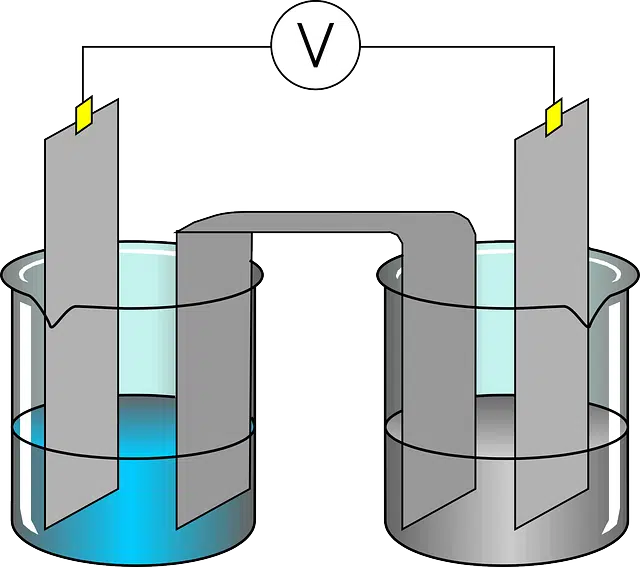
Electroplating is based on so-called electrodeposition . This treatment uses electric current to achieve the reduction of metal cations found in an aqueous solution, sedimenting them on a conductor and thus creating a film or layer .
What makes electroplating possible is the transfer of metal ions to a cathode from an anode . These ions are deposited in an aqueous solution at the cathode, where reduction via electrodeposition takes place.
Ions, cations, cathodes and anodes
As you can see, to understand what electroplating is, you have to know what various notions used in chemistry and physics refer to. First of all, we must take into account that an ion is an atom - or a group of atoms - that has an electric charge due to the gain or loss of one or more electrons.
An ion that has a positive charge, meanwhile, is called a cation . If the charge is negative, it is an anion .

Thanks to electroplating it is possible to cover rings with a layer of silver.
Cathodes , on the other hand, are negative electrodes: they carry out the reduction reaction that generates a decrease in oxidation. The anodes , on the other hand, are positive electrodes where the oxidation reaction that increases the oxidation state takes place.
Applications of electroplating
Electroplating uses different processes that are based on electrodeposition. It generally aims to optimize the mechanical properties of the treated elements, which can increase their resistance or gain hardness , for example.
Said colloquially, electroplating serves to deposit a metallic layer on an object . Thus, the object in question is covered with a metal film that can have different thicknesses .
Since an electrolytic medium is needed that contains the ions of the metal to be deposited, electroplating is also referred to as electrolytic plating or electroplating .
Functionality and aesthetics
Many times the use of electroplating is due to functional needs. However, it is also used for aesthetic reasons because metal plating can give shine to a ring , bracelet or other product.
Silvering is a procedure that can be achieved with electroplating. To silver is to cover with silver : therefore, to silver a metal is to cover it with a layer of silver. Electroplating makes this coating possible on copper , steel and other metals.
