Ionization energy is the energy needed to remove an electron in its ground state from an atom.
Ionization energy is the minimum amount of energy that an electron must absorb to escape nuclear influence . The concept is associated with the level of difficulty for an ion or atom to give up an electron.
It is possible to express the ionization energy in kilojoules per mole ( kJ/mol ) or in electronvolts . From this data, the reactivity of a chemical compound and the strength of its chemical bonds can be known.
Understanding ionization energy
To understand what ionization energy is, you must first know the meaning of several terms. An atom is a particle made up of electrons surrounding a nucleus. These electrons , in turn, are elementary particles that have a negative electrical charge .
An ion , on the other hand, is an atom or group of atoms that gains electrical charge by adding or losing electrons. In this framework, the idea of ionization refers to the conversion of atoms or molecules into ions.
With all this clear, it is easier to approach the idea of ionization energy. The notion refers to the minimum energy required to ionize an atom or molecule .
What this energy (also known as ionization potential ) allows is for an electron to no longer be under the influence of the nucleus, which can detach itself from the atom or ion in question.
It must be considered that, in its orbit around the atomic nucleus , the electron has a certain energy. The ionization energy is equal to the difference between the energy that the electron has in its orbit and the energy it has outside the atom.
The higher the ionization energy, the more difficult it is to remove an electron.
Classification according to type
It is possible to differentiate between different types of ionization energy. The first ionization energy is known as the energy needed to eliminate the first electron from an atom classified as neutral (that is, with the same number of electrons and protons). This first ionization energy is identical to the orbital energy of the electron, although with the opposite sign.
The second ionization energy , for its part, is the energy necessary to eliminate the second electron from the ion. Then other successive energies appear ( third ionization energy , etc.).
It should be noted that the elimination of the second electron is more complicated compared to the first, which implies that the second ionization energy has to be greater than the first. This trend in energy levels is maintained: the third ionization energy is greater than the second, the fourth is greater than the third, and so on.
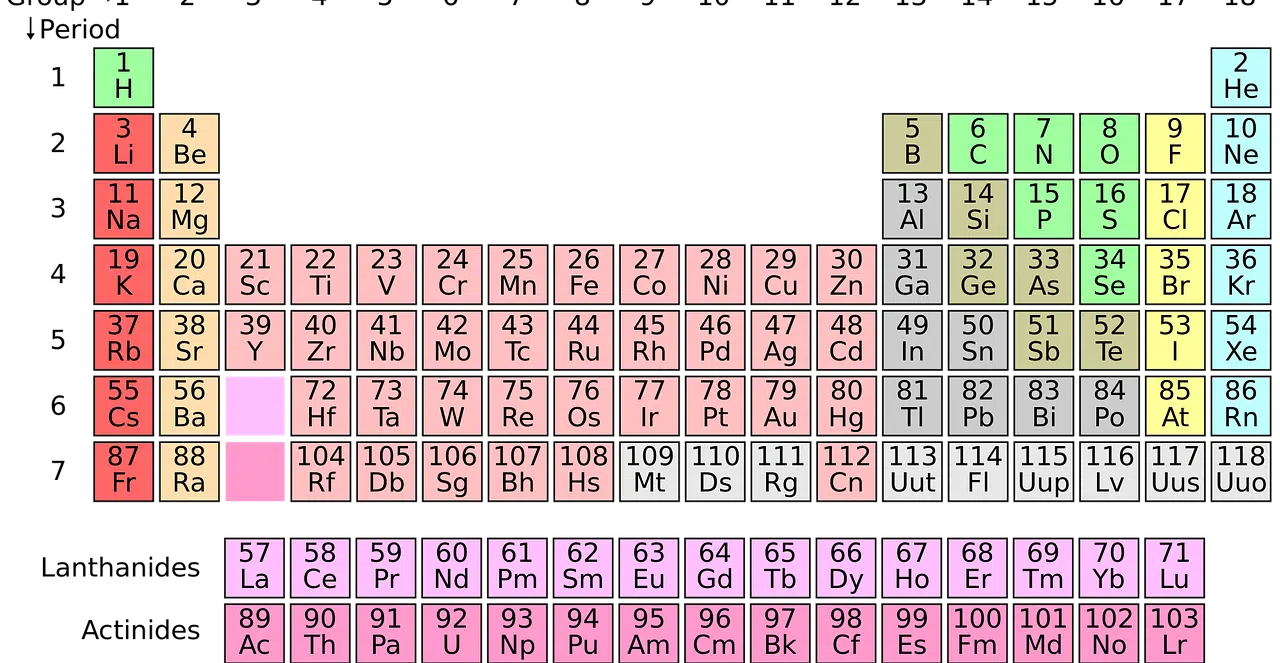
Observation of the periodic table allows us to obtain information about the ionization energy of the elements.
Factors influencing ionization energy
It should be noted that the higher the ionization energy, the more complicated it is to remove an electron. It should be considered, however, that various factors influence the forces of attraction .
The distance of the electron from the nucleus is one of them: the closer it is, the more attraction. On the other hand, if two electrons are present in the same orbit, the attraction exerted by the nucleus registers an alteration, with the ionization energy being lower in the paired electrons. The attraction is also less if there are more electrons between the nucleus and the outer shell.
On the other hand, the forces of attraction are stronger as the atomic number increases. Likewise, the ionization energy is higher if the electronic configuration is stable.
The periodic table of the elements
The periodic table is a scheme where each chemical element has its position according to its atomic number (that is, the number of protons it has in each atom), its electronic configuration and its chemical properties . In this way, elements that are located in the same column show similar behavior.
As we already indicated, the ionization energy increases with the atomic number and registers a decrease for higher energy orbitals.
Going from left to right on the periodic table, the ionization energy increases while the atomic radius decreases. Instead, a scroll from top to bottom in the table shows that the ionization energy falls because the number of layers in the elements increases progressively.
The trends are explained by the fact that, in the lower area of the periodic table, the elements have a higher number of orbitals, so the outermost electrons are far from the nucleus, they can be released more easily and there is a higher ionization energy. low. Furthermore, on the left side the elements have fewer protons, which means that electrons are also easier to lose.
How ionization energy is measured
Measurement of ionization energy is usually carried out using atomic spectroscopy . Spectroscopy is the analysis of the interaction between matter and electromagnetic radiation, which may include the emission or absorption of radiant energy.
In this case, the spectrum of light radiation that emits colors is taken into account, and through observation it is possible to establish the energy levels needed for each electron to be released from its orbit .
