Electrochemistry analyzes how electricity is generated from chemical combinations.
Electrochemistry is the branch of physical chemistry focused on the laws that refer to the generation of electricity through chemical combinations . Physical chemistry , meanwhile, is the science that analyzes the links between the chemical properties and the physical properties of a matter.
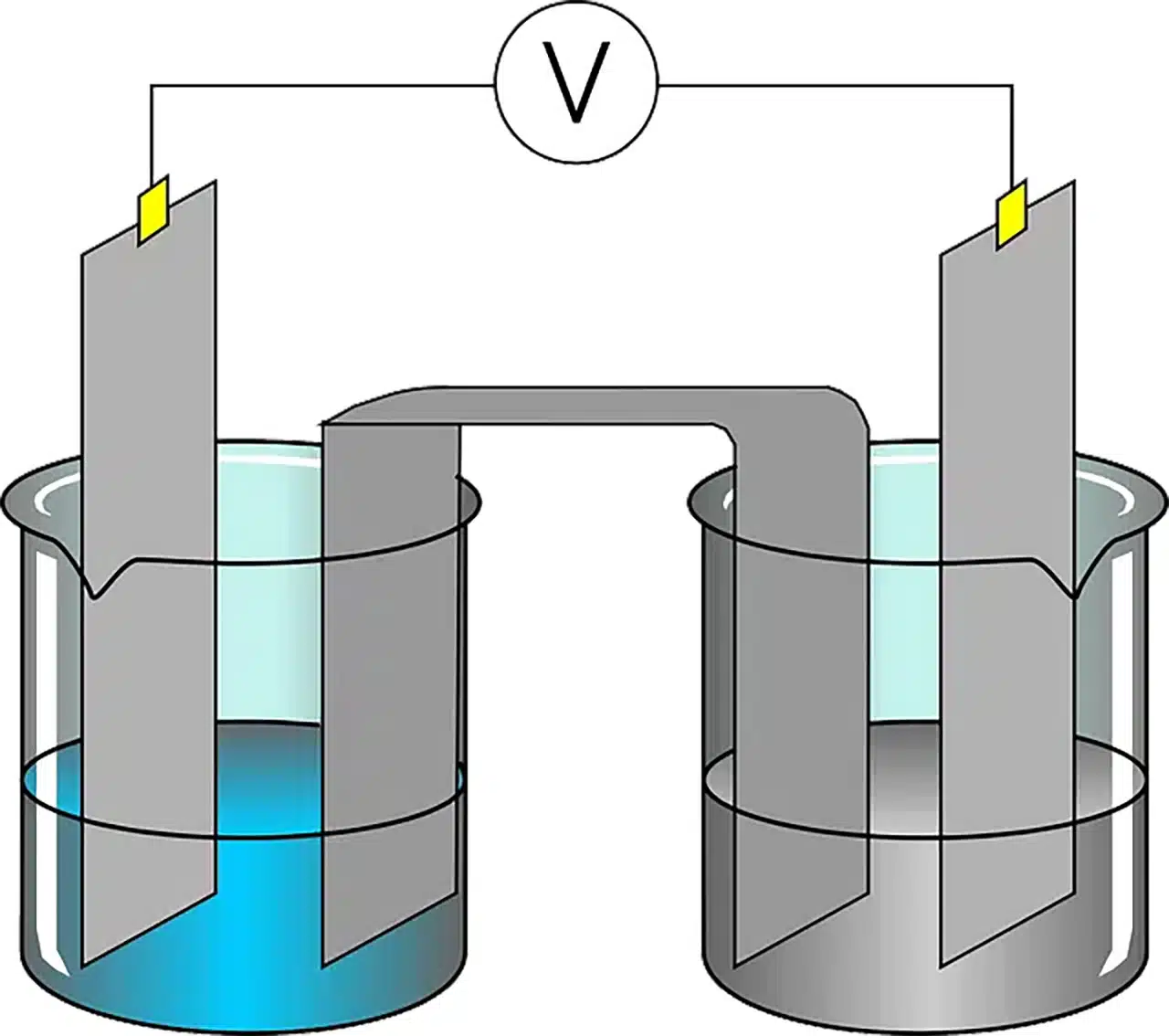
One of the purposes of electrochemistry is to understand the chemical reactions that occur at the interface of an electrode and an electrolyte . The electrode is the end of an electrical conductor that is in contact with a medium and that allows an electric current to be received or transmitted. The electrolyte , for its part, is the substance subjected to electrolysis (that is, a process of decomposition into ions caused by current).
Redox reactions
When there is a transfer of electrons between the molecules involved in a chemical reaction , it is a reduction – oxidation reaction ( redox reaction ). These reactions are key to the production of electricity.
The transfer that is generated in the redox reaction occurs between oxidizing elements and reducing elements. In this framework, energy is released and transformed into electricity.
History of electrochemistry
The history of electrochemistry dates back to discoveries and advances in the study of electricity and chemical processes . In the late 18th century, scientists began to explore the relationship between electricity and chemical changes. One of the first important milestones was the discovery of the voltaic cell by Alessandro Volta in 1800. The voltaic cell was the first device capable of generating a constant electric current through the chemical reaction between metals and conductive liquid substances.
In the 1830s, Michael Faraday performed numerous experiments and formulated the fundamental laws of electrochemistry. Faraday established the quantitative relationship between the amount of electricity passing through an electrolyte solution and the chemical reactions that occur at the electrodes. His contributions laid the foundation for the understanding of electrolysis and the development of electrochemical cells.
In the 19th century, electrochemistry found practical applications in industry and technology . Electroplating, the process of coating metal objects with a protective layer, became a common technique. Furthermore, obtaining aluminum through the electrolysis process of aluminum oxide allowed its large-scale production.
As the 20th century progressed, electrochemistry spread to fields such as energy and biology . The development of rechargeable batteries and fuel cells , which harness electrochemical reactions to generate and store energy, revolutionized portable technology and electric vehicles. Furthermore, electrochemistry played a crucial role in the advancement of neuroscience and the understanding of biological processes involving redox reactions.
Today, electrochemistry continues to be an active field of research and is applied in a wide range of areas, including electronic device manufacturing, renewable energy storage, chemical synthesis, and medicine . Understanding and controlling electrochemical reactions is critical to addressing environmental and energy challenges, and electrochemistry is expected to play an increasingly important role in the development of sustainable and technological solutions in the future.

Batteries are a type of electrochemical cell.
electrochemical cells
An electrochemical cell is a device that starts from a chemical reaction to obtain electrical energy or vice versa (when the incorporation of electrical energy produces a chemical reaction). These cells consist of two electrodes immersed in an electrolyte solution that allows the flow of ions. The electrode where oxidation occurs is called the anode , while the electrode where reduction occurs is called the cathode . During cell operation, electrons are released at the anode and travel through an external circuit , generating electrical current.
A voltaic cell or galvanic cell , in this context, is a type of electrochemical cell that acquires electrical energy thanks to the spontaneous redox reactions that occur inside it. The batteries are simple voltaic cells. Electric batteries , in turn, are made up of several voltaic cells connected in parallel or series.
