
Distillation allows the components of a mixture to be separated.
Distillation is the process and result of distilling . This verb refers to filtering or causing a liquid to drip , or to achieving the separation of one component from others through the application of heat .
For example: "The distillation of whiskey began to develop at the end of the 15th century" , "In the process of obtaining fuel, the distillation of petroleum is very important" , "The Chemistry teacher taught us what distillation consists of" .
Distillation characteristics
The notion of distillation - originating from the Latin word destillatio - is usually used to name the procedure that allows the separation of substances that form a mixture , through their condensation or vaporization .
Thanks to the different condensation and boiling points of substances, distillation makes it possible to distinguish between liquefied gases, solids that were dissolved, and liquids.
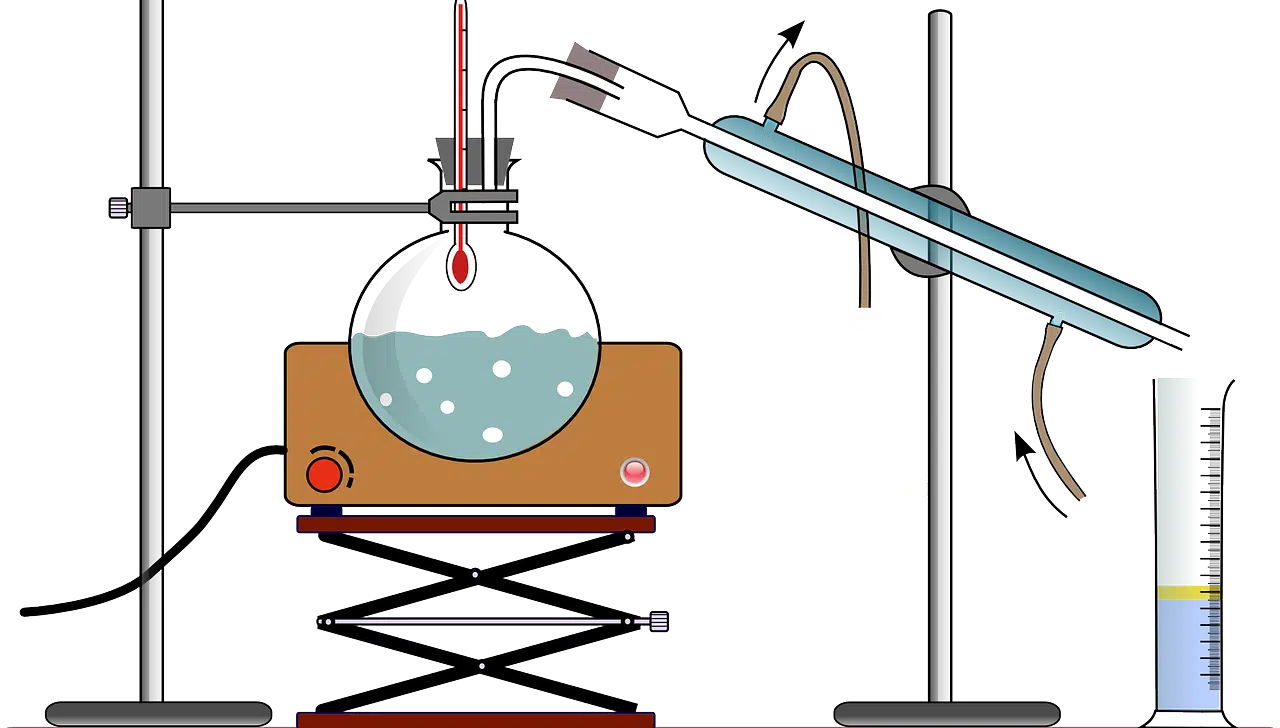
There are different types of distillation.
Classification according to type
Depending on how the process develops, it is possible to talk about different types of distillation:
Simple distillation
It is used when there is only one volatile substance in the mixture of liquid products , or when there is more than one but the difference in the boiling point of the liquid with the highest volatility with that of the rest equals or exceeds 80 °C. This procedure results in a single product, since: it is the amount of components that were in the original mixture; one of the components was considerably more volatile than the rest. To carry out simple distillation, you must have a vacuum adapter and a vacuum system.
Within this type of distillation, we can distinguish between the following two classes:
- At atmospheric pressure : carried out at ambient pressure . It is mainly used in cases in which the boiling temperature of the product is lower than its chemical decomposition temperature.
- At reduced pressure : it is achieved through a decrease in pressure with the aim of lowering the boiling point of the component that we wish to subject to distillation. It is usually used when the boiling point of the product is higher than the temperature of its chemical decomposition.
Fractional distillation
It is used when the boiling points of the volatile substances in the liquid mixture have a difference of less than 80 °C. When the mixture is heated, the vapor becomes richer in the element with greater volatility, a property that is used to divide the liquid compounds. This type of distillation is mainly characterized by requiring a fractionation column. It can be carried out under reduced or atmospheric pressure, as occurs with simple distillation.
Steam distillation
It is used to purify or isolate compounds whose boiling point is very high, through the use of temperatures that do not exceed 100 °C. This type of distillation is very convenient for dealing with substances that have a boiling point well above 100 °C and that never decompose beyond this temperature.
Thanks to steam distillation it is possible to separate substances that are not soluble in H2O, as well as those with a slight volatility from other non-volatile ones. An excess of water must be added to the mixture in which the product to be separated is located. For this procedure, two flasks are used (crystal or glass vessels whose shape is usually spherical and ends in a straight, narrow cylinder): the distillation flask, in which the hot water-soluble and/or volatile compounds remain; and the collector , which recovers the water-insoluble and the volatile ones. To isolate the organic compounds from the collecting flask, an extraction is carried out.
