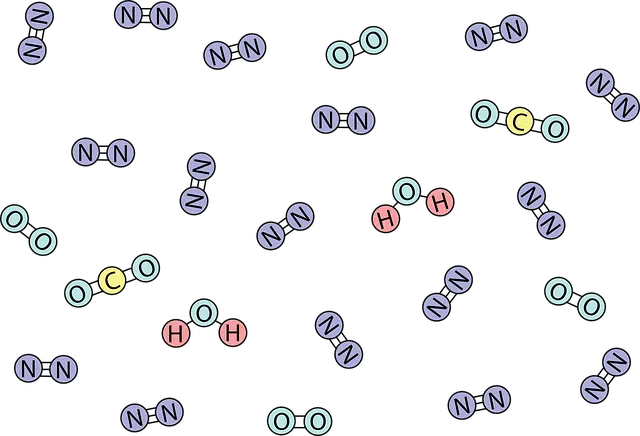
Covalent is that bond established between atoms that have shared pairs of electrons.
The adjective covalent is used in the field of chemistry to describe the bond that is generated between atoms that have shared pairs of electrons . Something that has at least one covalent bond is also described as covalent.
It is important to remember that particles that have an electrical charge and are formed by a molecule or an atom that is not neutral are called ions . Ions, according to the octet rule stated by the American Gilbert Newton Lewis in 1916 , have the tendency to use eight electrons to complete the last energy levels and thus achieve stability in their configuration.
Atoms , to respect the octet rule, can use different types of chemical bonds to join together. Among them appears the covalent bond , which involves the sharing of electrons in the last level . This type of bond requires that the difference in electronegativity registered between the atoms be less than 1.7 .
Development of a covalent bond
Covalent bonds develop between atoms of different non- metal elements and between atoms that belong to the same non-metal element. Covalently bonded atoms share their electron pairs in the molecular orbital .
These atoms can share between one and three pairs of electrons in a covalent bond: therefore, the bonds can be single , double or triple depending on the case. If the bond occurs between equal atoms that have a difference in electronegativity of less than 0.4 , a nonpolar covalent bond is obtained. On the other hand, if the bond is developed by atoms of different elements that have a difference in electronegativity greater than 0.4 , it is a polar covalent bond .
Covalent bonds can be polar or nonpolar.
Main aspects
According to chemists G. William Daub and S. Seese , in any covalent substance (such as a hydrogen molecule) the following four aspects can be seen:
* If observed individually, that is, outside of a combination, atoms have very different properties than those exhibited by molecules. For this reason, when writing the chemical formula of hydrogen, for example, we must put a two as a subscript of H , since it is a diatomic molecule (one that is made up of two atoms, whether or not they are of the same chemical element ). ;
* The two electrons are attracted to the two positive nuclei, something that occurs with the aim of producing a more stable molecule than one in which the atoms are separated. This causes a covalent bond to be generated. Since the attraction to which the nuclei subject the electrons manages to cancel the repulsion between them, there is a good chance of finding electrons between the two nuclei;
* The distance between the nuclei must allow the 1s orbitals to have maximum overlap. For example, this value in the hydrogen molecule is around 0.74 angstrom. If this is not true, then we speak of bond length to define the distance between two covalently bonded atoms;
* 52 kilocalories are necessary to cut the covalent bonds that exist in 1 gram of hydrogen gas.
Types of covalent substances
With respect to covalent substances, it is possible to recognize the following two:
* molecular covalents , that is, the bonds that form molecules with low boiling and fusion temperatures, insulators of heat and electric current, soluble in polar or non-polar solvents (depending on whether the molecules themselves are polar or non-polar), such as benzene, nitrogen, oxygen and carbon;
* lattice covalents , crystalline networks with an indefinite number of atoms, similar to ionic compounds, characterized by being very hard, insoluble and with high boiling and fusion temperatures, such as diamond and quartz.
