The electronic configuration is linked to the way in which electrons are organized in an atom.
Before proceeding to know the meaning of the term electronic configuration, it is necessary to discover the etymological origin of the two words that give it its shape:
– Configuration , first of all, comes from Latin. Exactly it derives from configuratio , which can be translated as "action and effect of giving a shape using several parts." It is the result of the sum of the following components: the prefix con- , which means "together"; the noun figura , which is synonymous with "form"; the ending -ar and the suffix «-cion», which is synonymous with «action and effect».
– Electronics , secondly, is a word that comes from Greek. It is the result of the union of these components: the noun elektron , which means both "amber" and " electricity ", and the suffix -iko , which is used to indicate "relating to."
What is electron configuration
The notion of configuration refers to the organization of the various elements that make up something, determining its characteristics and its shape. Electronics , for its part, is the analysis and application of the properties of electrons in different media.
We must also remember that electrons are elementary particles that have a negative electrical charge. These particles revolve around the nucleus of the atom .
From these definitions we can understand what the concept of electronic configuration alludes to. This is the way in which electrons are arranged in an atom according to the electronic shell model . The series of orbits followed by the electrons that revolve around the nucleus of the atom is called the electronic shell .
In the same atom, different orbits around the nucleus can be recorded. The electronic configuration refers to how the electrons are arranged in these orbits , which imply different energy levels . The further the electron is orbiting from the nucleus, the higher its energy level. That is to say: electrons in an electron shell far from the nucleus have a higher energy level than electrons in closer electron shells.
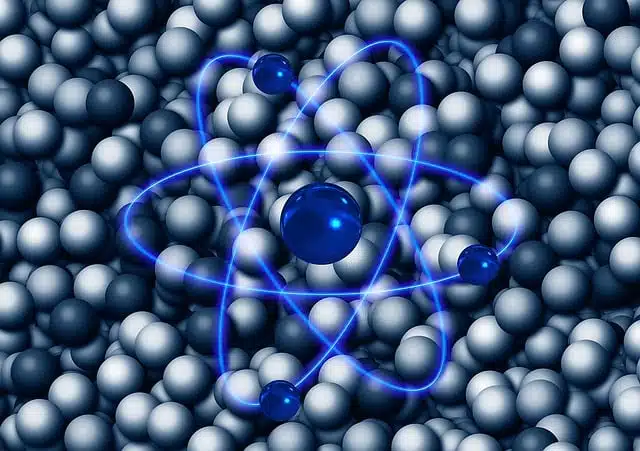
The electronic configuration refers to how the electrons are arranged in the orbits they make around the nucleus.
The chemical combinations of the atom
An atom, in this framework, can establish certain chemical combinations according to its electronic configuration. This configuration also determines its location on the periodic table of elements.
Up to seven energy levels, also called energy orbitals, can be found around the nucleus, which are numbered from 1 to 7 . Electrons with the lowest energy level orbit in level 1. Each of these seven levels, on the other hand, can be subdivided up to four times: these sublevels are called s , p , d and f . These numbers and letters are used for electron configuration notation.
In addition to everything indicated, we cannot ignore the fact that in each sublevel there is a maximum number of electrons that can exist. Thus, for example, in the s sublevel there can only be a maximum of 2 electrons while in the p sublevel there can be no more than 6.
In the same way, in the d sublevel a maximum of 10 electrons is established and in the f sublevel there can be no more than 14 electrons.
When establishing the electronic configuration of an atom, it will be necessary to know not only the number of electrons it has but also to know how to place them in the different energy levels and respect the maximum electron capacity of each sublevel.
