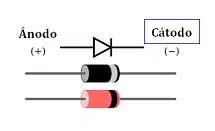
 The notion of cathode is used in the field of physics to refer to a negative electrode . The etymology of the term refers us to the Greek word káthodos , which translates as "descending path" .
The notion of cathode is used in the field of physics to refer to a negative electrode . The etymology of the term refers us to the Greek word káthodos , which translates as "descending path" .
The end of an electrical conductor that collects or transfers a current when in contact with a medium is called an electrode. In the specific case of cathodes, they are electrodes that have a negative electrical charge .
The ends or terminals of a battery or cell are called poles , which can be negative or positive. This quality is called polarity . The direction of flow of electric current was conventionally set as the flow of charges that goes from the positive pole to the negative pole.
In devices that provide power , such as batteries, the cathode has positive polarity . On the other hand, if the element consumes energy, the cathode has negative polarity .
Redox (reduction-oxidation) reactions are generated in the cathodes that cause a material, by obtaining electrons (elementary particles that have a negative charge), to undergo a reduction in its oxidation state. At the anodes (positive electrodes), on the other hand, oxidation reactions take place, which lead to a material losing electrons and increasing its oxidation state.
With respect to etymology, it is known that the term was coined by the physicist and chemist Michael Faraday , originally from Great Britain, who made great contributions to the fields of electrochemistry and electromagnetism. More specifically, Faraday first mentioned it in the context of his experimental investigations into electricity , in the seventh series.
The meaning he gave to the word cathode was "exit, downward path", since its origin is found in a Greek word that can be translated as "path, downward"; in this case, it should be understood only in reference to the electrolyte of the electrochemical cells .
 An electrode that, based on the thermionic effect generated by heat , emits electrons is called thermionic cathode ; This phenomenon is also known as the Edison effect . This type of cathode, for example, is the electron source used in thermionic valves.
An electrode that, based on the thermionic effect generated by heat , emits electrons is called thermionic cathode ; This phenomenon is also known as the Edison effect . This type of cathode, for example, is the electron source used in thermionic valves.
One of the most important properties of the thermionic cathode is that it can increase its own temperature on its own; To do this, it causes a heating current to circulate through it, or uses a filament to which it is thermally coupled. Materials that manage to emit electrons at a temperature that is not too high are the most efficient to take advantage of the thermionic effect; Some of the most common are alloys of tungsten (also called tungsten ), thorium, and lanthanides; Another option is to coat the cathode with calcium oxide.
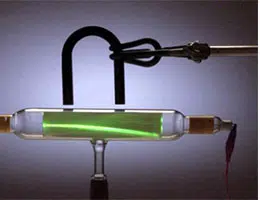
Cathode rays , on the other hand, are known as the electron currents that can be observed in vacuum tubes, those that are made of glass and that are equipped with a minimum of two electrodes, an anode and a cathode in a configuration that is called a diode . When the cathode heats up, it emits radiation that moves in the direction of the anode; If the internal glass walls behind the latter have a coating of some fluorescent material, then they produce an intense glow.
This concept is found in most television and monitor screens of past decades, as they used cathode ray tubes , a technology that constantly emits rays towards a glass screen covered in lead and phosphorus to reproduce images. Lead protects the person from lightning radiation while phosphorus makes image reproduction possible.
