Butanol is a primary alcohol
A primary alcohol
To understand what butanol is, then, it is necessary to first analyze the concept of butane. We said that it is a hydrocarbon : that is, a compound that is generated when carbon combines with hydrogen. In the specific case of butane, it has four carbon atoms .
Returning to the idea of butanol, it is an alcohol derived from butane. An alcohol is a compound that has a hydroxyl group (-OH) that replaces a hydrogen atom and that is attached to a saturated carbon atom through a covalent bond.
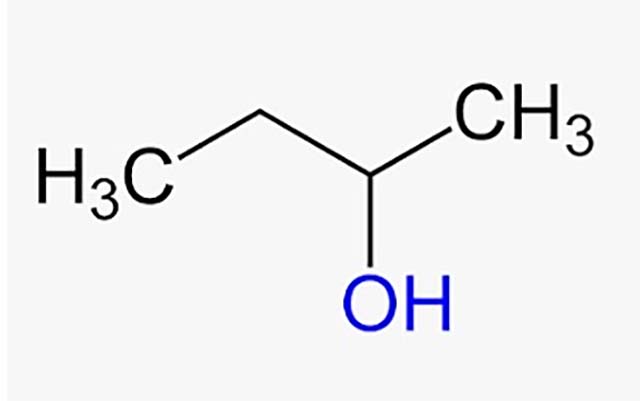
Butanol, or more precisely 1-butanol ( butan-1-ol ), is a primary alcohol (the hydroxyl group is attached to a primary carbon) whose formula is H3C-(CH2)3-OH . In nature, butanol appears as a byproduct of the fermentation of certain carbohydrates .
Among the uses of butanol, this alcohol is used to produce butyl acrylate and as an artificial flavoring. That is why it can be found in candy , ice cream, drinks and other food products.
2-butanol
2-Butanol or butan-2-ol , on the other hand, is a secondary alcohol that, unlike 1-butanol, is not mentioned as butanol but always including the number. The formula of this compound is H3C-CH2-CH(OH)-CH3 .
On the other hand, we can also mention its isomers, which are butan-1-ol , methylpropan-1-ol and methylpropan-2-ol . Isomers respond to the concept of isomerism , a property that chemical compounds have whose molecular formula has the same relative proportions of the atoms of its molecule, although different chemical structures, configuration and properties.
Biobutanol
It should be noted that plant-based butanol or biobutanol is currently promoted as fuel . This alcohol is similar to ethanol , but has two additional carbon atoms.
According to experts, butanol of plant origin offers 95% of energy compared to an identical volume of gasoline. Furthermore, it is possible to mix this butanol with gasoline in a higher proportion than ethanol.
Health effects
If butanol is inhaled, it can cause poisoning, although the chances are slim given its low volatility. They mainly irritate the upper respiratory tract and cause consequences such as cough, drowsiness, dizziness, headache and breathing problems. If it is absorbed into the blood, then its effects may be similar to those of ingestion (detailed below).
Ingestion of butanol can cause nausea, pain in the abdomen and head, diarrhea and dizziness. If ingested in considerable doses , it can also attack the liver, kidneys and hearing system. To cause death, between 3 and 7 ounces must be consumed. Then we have the effects that it can have when coming into contact with the skin, which are summarized in a decrease in natural oils and some of the symptoms just mentioned.
If butanol touches your eyes, it can inflame them and cause difficulty seeing clearly. If contact occurs through butanol vapor, the consequences vary between pain, tearing and irritation.

Contact with butanol can cause dizziness, blurred vision and other symptoms.
First aid
In case of inhalation, it is recommended to take the person to fresh air so they can breathe. If you cannot achieve this or if it is difficult, you should administer oxygen and contact a doctor . Ingestion can be combated by inducing vomiting immediately; If it is combined with loss of consciousness, it is important not to try to administer anything by mouth.
If butanol has come into contact with the skin, it is necessary to wash it with plenty of water for at least a quarter of an hour, in addition to removing any clothing that has been contaminated and washing it well before using it again. The wash is also applied to the eyes, without forgetting the eyelids.
