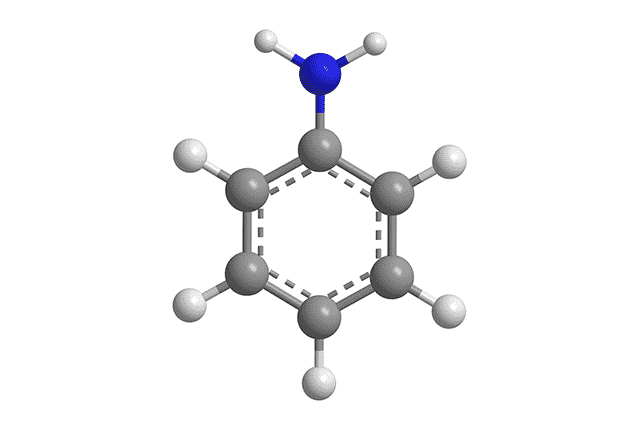
The chemical formula of aniline is C₆H₅NH₂
The Portuguese word anil , which can be translated as “indigo” , came into German as Anilin , in turn derived from the French aniline . The concept arrived in our language as aniline .
an amine
Aniline is an amine : that is, a substance derived from ammonia (a gas formed with one nitrogen atom and three hydrogen atoms). This organic compound is used in different ways in the industrial field.
Aniline is generally used as a dye and to make paints . Herbicide development, rubber production, and explosives manufacturing can also be carried out with the use of aniline. It should be noted that the molecules can dissolve in numerous organic solvents, are slightly soluble in water and do not evaporate quickly at room temperature.
History
The British chemist William Perkin (1838-1907) was the one who warned that aniline, through its oxidation and dilution, allowed synthetic dyes to be obtained. The discoverer of aniline, however, was the German Otto Unverdorben (1806-1873), whose discovery took place in 1826 , three decades before Perkin 's experiments.
It all began throughout the Middle Ages, when the European continent gradually became a power in terms of maritime business, until it finally dominated these relations completely. It did not take long until all the dyes came from America or India, and given the high shipping costs and the impossibility of obtaining the raw material in Europe, chemists began to investigate ways to replace them synthetically. But only in the 1800s did scientific advances allow them to create these dyes that would change the European situation.
Then came 1826, when Unverdorben managed to isolate aniline for the first time, destructively distilling indigo; At that time, he named it crystallin . Eight years later, the also German Friedlieb Runge managed to isolate a substance from coal tar (a highly viscous black or brown liquid whose odor resembles mothballs), resulting in a bluish color when treated with calcium hypochlorite to which called cyanol .
At the beginning of the following decade, Carl Julius Fritzsche used caustic potash to treat indigo and obtained an oil that he called aniline , starting from a plant from which indigo is also obtained, Indigofera suffruticosa . Shortly afterwards, Nikolay Nikolaevich Zinin came across the base benzidam , reducing nitrobenzene. In 1843, August Wilhelm von Hofmann proved that they were dealing with a single substance, aniline, and it was then that they began to use this name for all of them.
Toxicity and risks
It is important to emphasize that aniline is toxic and can cause various health problems through inhalation, ingestion or even contact. This is because aniline causes damage to hemoglobin .
Headache, dizziness, skin irritation and seizures are some of the disorders that aniline can cause in people. Given that aniline comes in many forms, both concentrated and in its physical state, and that it is part of many different processes, there is no single regulation that regulates its use, although broadly speaking it is possible to state that its ingestion is not recommended. inhalation or direct contact, nor being exposed to it for a long time.

Aniline dye powder of various colors
Some recommendations to reduce risks, before the inevitable visit to a health center:
* if inhaled, go outdoors to breathe fresh air;
* If it touches the skin, remove contaminated clothing and wash the skin with plenty of soap and water ;
* If it touches your eyes, wash them with water. Contact lens wearers should remove them immediately;
* if ingested, induce vomiting and wash mouth.
