Aldehydes are dehydrogenated alcohols.
Aldehyde is a term that derives from the English word aldehyde . This word, in turn, is an acronym for a Latin expression: alcohol dehydrogenatum . In this sense, we can state, therefore, that it comes from an expression such as "dehydrogenated alcohol."
Aldehydes are organic chemical compounds that arise when certain alcohols are oxidized .
As the aforementioned Latin expression suggests, an aldehyde is a dehydrogenated alcohol : that is, one that has lost hydrogen atoms. Aldehydes have a formyl functional group, which is formed when a hydrogen atom is separated from the compound known as formaldehyde.
Nomenclature of aldehydes
To name aldehydes, the – ol ending in the name of the hydrocarbon is eliminated and -al is added.
Thus, according to the nomenclature of the International Union of Pure and Applied Chemistry , one can speak of aldehydes such as methanal (or formaldehyde , according to the trivial nomenclature), ethanal ( acetaldehyde ) or propanal ( propaldehyde ), for example. Name the simplest ones according to their carbon number.

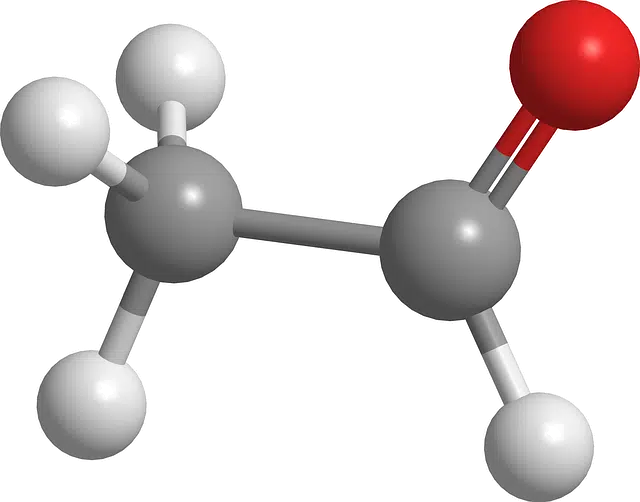
Formaldehyde or methanal is part of the group of aldehydes.
Uses of these chemical compounds
Aldehydes can act as reductants . In this way, they are used in different industries to produce paints, dyes, solvents and plastics, among other elements. It can also be used as a preservative and to obtain compounds such as melamine or bakelite .
No less important is that, in addition to what has been explained so far, they can also be used for other equally significant uses:
-To carry out the creation of various types of explosives such as the so-called TNPE.
-To create technical plastics that are commonly used to carry out what is the replacement of metal parts in both machinery and automobiles.
-In the same way, it should not be overlooked that they are also used within the field of perfumery.
It is important to mention that aldehydes can be formed through various natural processes. When a person drinks a large amount of alcoholic beverages and then feels the discomfort known as a hangover, this sensation could be due to the acetaldehyde that is produced during metabolization, according to some studies on the subject.
Other characteristics of aldehydes
In addition to everything indicated, we can highlight another important series of data about aldehydes, such as the following:
-The formula that, as a general rule, identifies them is this: CnH2n+1CHO.
-The boiling point that the aforementioned aldehydes have, which must be emphasized, is above that of alkanes, but is lower than that of alcohols.
-Among those that end in -al, we can highlight some such as ethanal, propanal, methanal or butenal.
-When there are two groups of aldehydes, they are given the ending "-dial". This would be the case of pentodial or butanedial.
-Specifically, it is determined that the most frequent ways or processes through which aldehydes are formed are carbonylation, dehydrogenation and oxidation of alcohols and also what is known as oxidation of alkyl halides.
