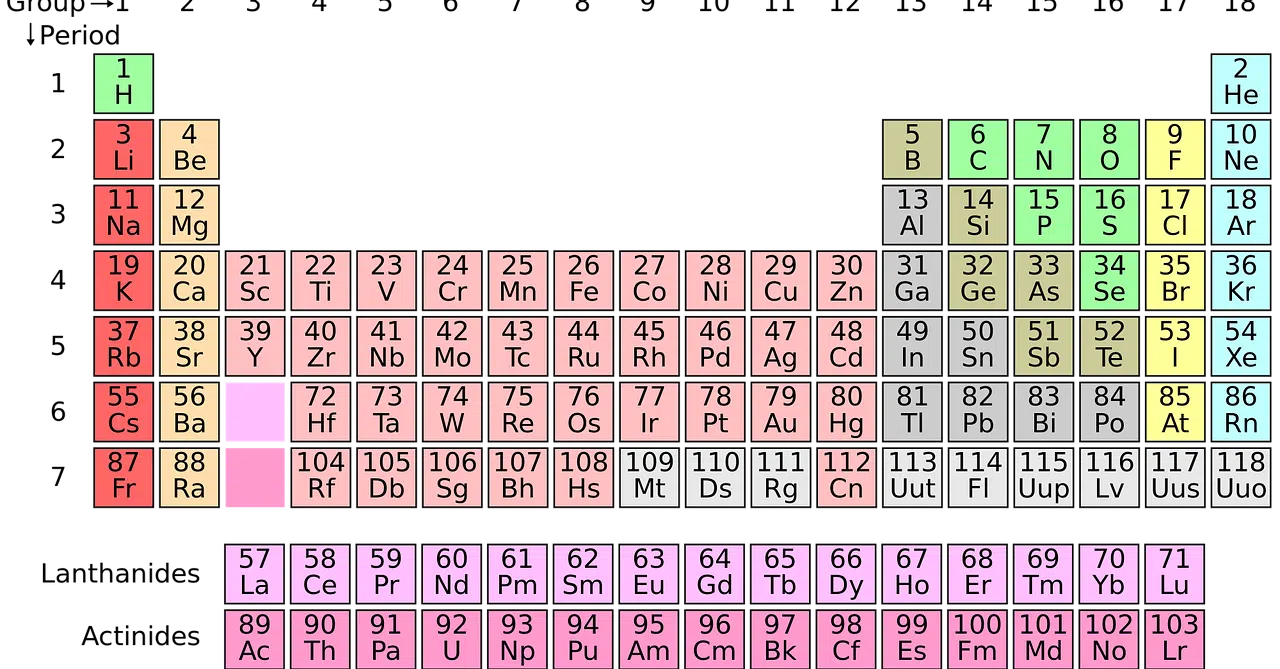
As one moves through the periodic table it is possible to observe the variations that show the electronic affinity of the different elements present.
Electronic affinity is the expression used by Chemistry experts to account for the energy that is released every time an atom that is neutral and in a gaseous state accepts at least one electron and, thanks to this, is formed into an ion of character. mononegative .
This process, also known as electroaffinity , is essential to understand the chemical behavior of the elements that are part of the periodic table . In this regard, it is important to note that this phenomenon, which can be exothermic or endothermic, does not develop identically in all cases because each chemical element has its particularities. As a consequence, there are both positive and negative values for electron affinity .
It must also be taken into account that the electron affinity (AE) increases in case of growth in the atomic number , the screen effect does not reach sufficient power or the dimensions of the atom decrease. As in the case of electronegativity (the property that is conceived as equivalent to electronic affinity taking into consideration an isolated atom), it is observed that in left-right orientation and from below in an ascending line, the electronic affinity increases.
Applications and scope of electron affinity
Electron affinity has applications in various disciplines and scientific areas, achieving a wide scope and diverse uses.
In the field of Chemistry , specifically, it is necessary to discover the interaction of atoms and how a chemical bond is formed.
Learning about this is key to identifying and analyzing a chemical reaction , to progress in the mastery of intermolecular forces and to incorporate enriching notions regarding the structure of a compound. When the electron affinity is high, say scholars on the subject, the atom is usually more prone to the admission of electrons , tending to form compounds of a stable nature. On the other hand, an electron affinity of low absolute value leaves the atoms more exposed to the loss of electrons, generating an ionic compound in this framework. In this sense, when wanting to determine the disposition of an atom to form an ionic bond or a covalent bond, it is essential to focus on electronic affinity .
Electroaffinity usually accompanies the variation evidenced by the so-called ionization energy : when this is low and the atom easily gives up an electron , the electronic affinity is also low and vice versa, that is, if there is a lot of ionization energy (gaining electrons in instead of losing them) the electron affinity will also be high.
Electroaffinity or electronic affinity is the flow of energy that ends up being released by an atom that is in the gas phase and isolated in the process of forming an ion.
It is interesting to know that, if we focus our attention on a group, it is revealed that, as we go down, the electronic affinity is lower but the atomic radius is higher. In a period, the advance translates into a decrease in the atomic radius and, in this scenario in which the attraction of electrons becomes increasingly stronger, the electronic affinity increases as we move within the period.
Judging by recent research and findings, electron affinity is gaining relevance within inorganic chemistry (when creating molecules with specific properties and synthesizing organic compounds ), quantum physics and technological innovation . Nor can we minimize the significance that this process achieves in projects for the manufacturing of semiconductor materials or in the production of increasingly effective drugs.
Periodic table and electroaffinity
By learning to interpret and understand what the periodic table of elements consists of and what value or meaning each of the data contained in it has, the process of understanding and identifying the importance and distinctive features of electron affinity is easier.
As we mentioned above, the variations that EA presents in periods and groups are notable and clear.
Starting from the left in a period and moving to the right, as the size of the atoms decreases and the effective nuclear charge increases, the electrons are attracted more strongly towards the nucleus, increasing in this framework the electronic affinity .

Electron affinity is useful in the fields of technological innovation, inorganic chemistry and quantum physics, among other areas. It is taken into account, for example, to develop drugs and materials with semiconductor properties.
When, when going through a group, the point of interest decreases, elements are found with atoms of increasingly larger size and less attraction of electrons towards the nuclei: in this scenario, the electronic affinity decreases .
Given that the measurement of this variable is complicated and that there are various factors that influence it, it is not possible (at least for the moment) to know exactly the electronic affinity of each of the chemical elements distributed in the periodic table . It has been determined, by sharing specific references, that metals have a higher electron affinity compared to that of non-metals.
