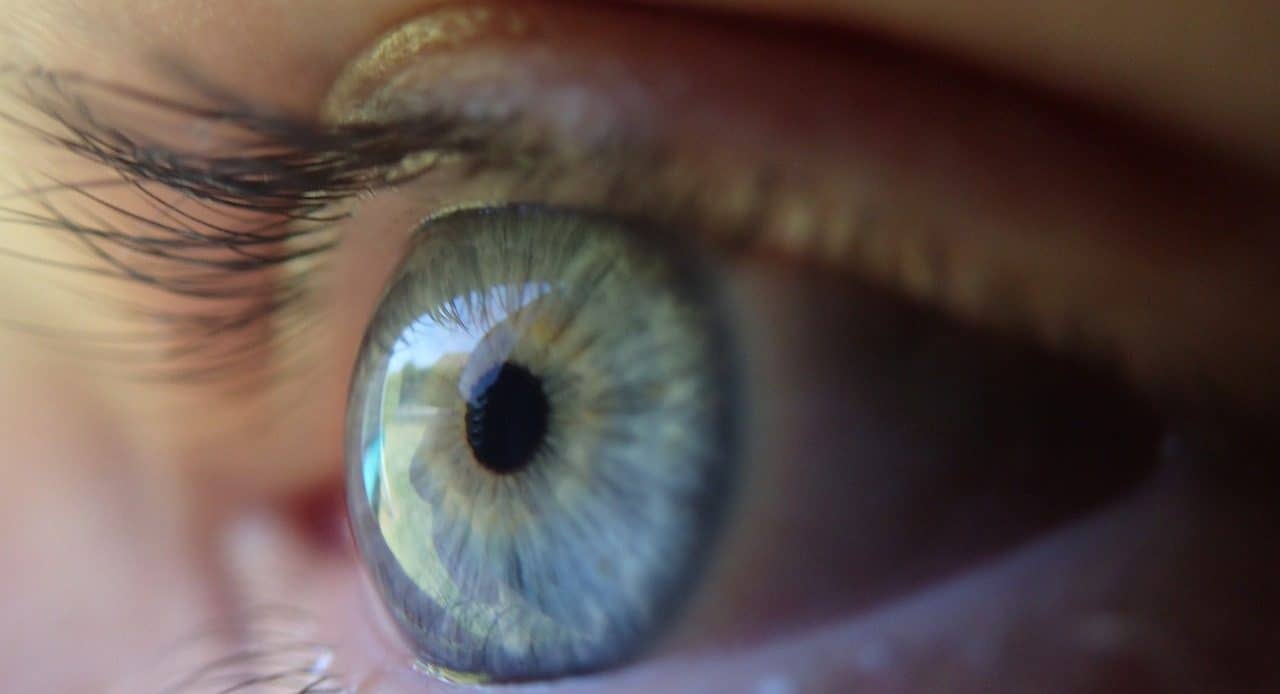
Aqueous humor is a liquid that is present in the eyeball.
Watery is an adjective that refers to something that is made up of water or has a large amount of it. It also alludes to what water looks like.
Before moving forward it is important to discover the etymological origin of the term. In this case, we can say that it is a word that derives from the Latin aquosus , which is the result of the sum of two components:
- The noun aqua , which can be translated as “water” .
- The suffix -osus , which is equivalent to “abundance” .
The aqueous humor
Aqueous humor , for example, is a liquid found in the eyeball , in front of the lens. This substance allows oxygenation and nutrition of the aforementioned lens and cornea, which lack blood supplies.
Other functions of the aqueous humor are to provide nutrients to the lens and cornea and to help light refraction reach the retina. It also helps different structures remain in position .
glaucoma
It is important to mention that certain changes in the aqueous humor cause eye diseases . The increased pressure of this fluid causes glaucoma , which affects the field of vision.
We have to explain that this pathology is identified because there is a gradual loss of the nerve fibers of the optic nerve . That is, the person who suffers from it not only begins to experience notable changes in the visual field but, little by little, loses sight.

Brine is an aqueous solution.
aqueous solutions
Aqueous solutions , on the other hand, are those that are formed with water . This means that water acts as the only solvent or, in certain cases, as the main solvent.
It should be remembered that a solution is a homogeneous mixture that is formed when a solute is dissolved in a solvent . If the solvent is water, it is called an aqueous solution.
There is an immense amount of substances that are soluble in water. That is why it is common for water to be called a universal solvent and for aqueous solutions to be very numerous.
We can determine that aqueous solutions receive that name because they involve two solvents, water being the most relevant (the one that is present in greater quantity). In the same way, we must not forget that they are used daily in laboratories and even in industries, especially those that revolve around chemistry.
Although we have mentioned that water is a universal solvent, we must keep in mind that there are substances that dissolve better in water than others. Thus, for example, we can emphasize that the most soluble are the so-called ionic ones, such as sodium chloride . On the other hand, the least soluble are the covalent substances, among which we can highlight insoluble metals.
Brine is an example of an aqueous solution. It is table salt that is dissolved in water, obtaining a solution that can be used for gastronomic purposes.
