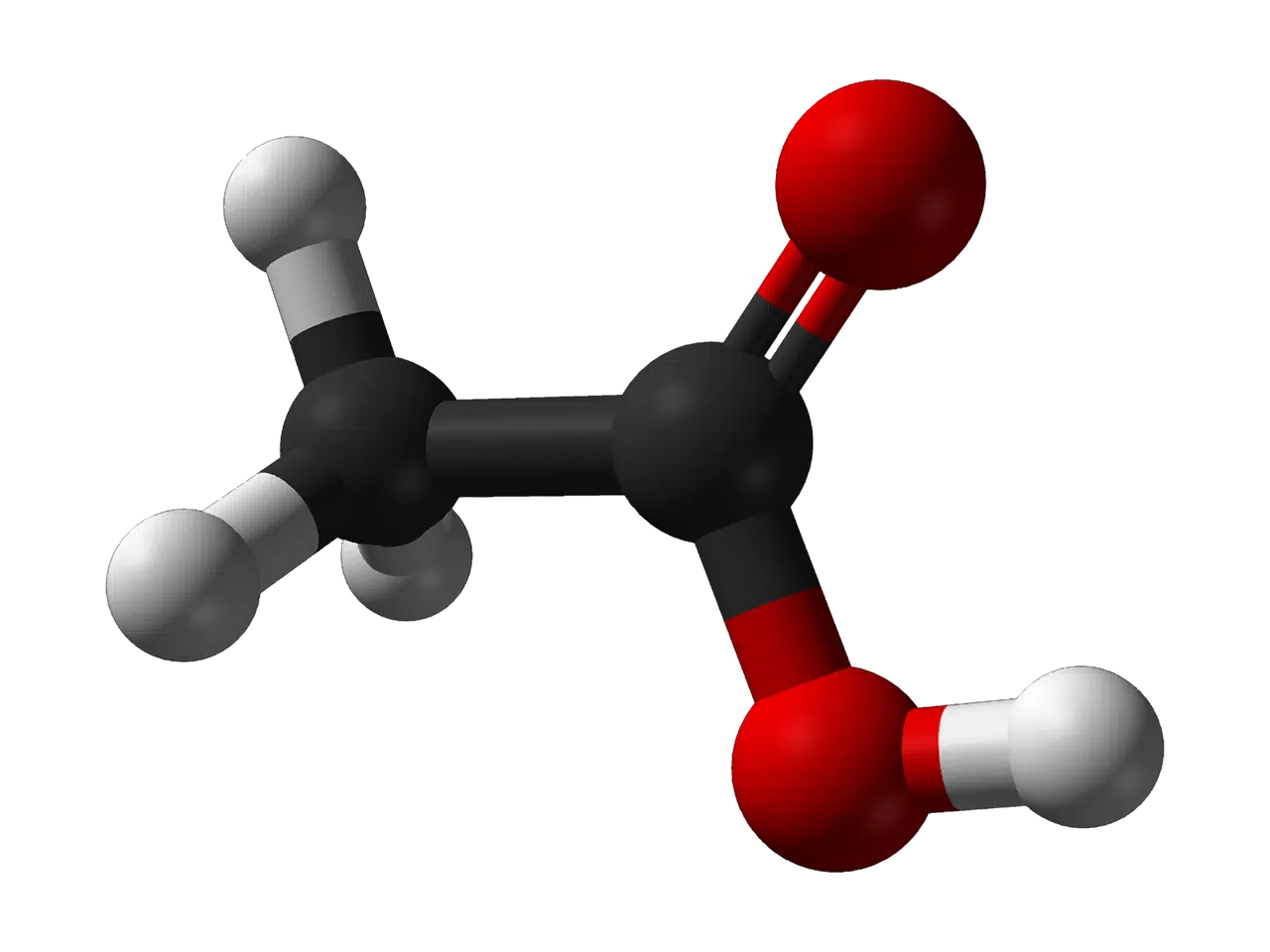
Acetic acid is called ethanoic acid according to the IUPAC.
Acetic acid is a chemical compound made up of carbon , hydrogen and oxygen . It is an organic substance that is also called ethanoic acid or methylcarboxylic acid .
This liquid, which lacks color and is characterized by its pungent odor, is generated when ethyl alcohol or ethanol oxidizes. It can be obtained through fermentation or through chemical synthesis .
Characteristics of acetic acid
Like all organic compounds, acetic acid has carbon among its constituents. Each of its molecules has two carbon atoms, in addition to four hydrogen atoms and two oxygen atoms, so its molecular formula is C2H4O2 .
Acetic acid is a carboxylic acid since it has a functional group called a carboxy group or carboxyl group ( -COOH ). In the specific case of this substance, its semi-developed formula is CH3-COOH . By having a two-carbon chain, it is in second place among the simplest carboxylic acids, only behind formic acid , which has a single carbon. The CH3 chain, meanwhile, positions it in the methyl group.
Another classification of acetic acid is as a weak acid . This is because, in an aqueous solution, it is not completely dissociated. In these cases of dissolution, it can record the loss of the proton of the carboxy group, resulting in acetate (its conjugate base). It should be noted that, according to the Brønsted-Lowry theory , an acid is a substance that can transfer protons, while a base is one that can accept them.
With a moderate acidity reaching 4.8 pKa , acetic acid has a boiling point of 117.9 ºC . If we focus on the melting point , it is located at 16.6 ºC .

Acetic bacteria can produce acetic acid.
Obtaining methods
There are several methods for obtaining acetic acid. Acetic or oxidative fermentation is that produced by Acetobacter bacteria, which are aerobic. In this procedure, we start with ethyl alcohol.
What these bacteria do is develop the production of vinegar through the fermentation of wine, cider , potatoes, rice or malt, among other inputs. It is estimated that vinegar, as a condiment, was created accidentally as a consequence of fermentation failures when trying to make wine: when the must is subjected to high-temperature fermentation, bacteria of this genus act on the yeast of the grapes.
Acetic acid, on the other hand, can also be obtained by anaerobic fermentation with the intervention of Clostridium bacteria. In this process, ethyl alcohol is not used as an intermediate, but rather the bacteria develop a chemical reaction in sugars .
From catalysis , it is also possible to produce acetic acid through the oxidation of acetaldehyde or ethanal carried out by oxygen . Acetaldehyde, in turn, is generated by the hydration of ethylene or by the oxidation of light naphtha or butane .
Carbonylation of methanol , finally, is the most common method to obtain acetic acid. It consists of promoting the reaction of carbon monoxide and methanol (methyl alcohol), using methyl iodide as an intermediate and a catalyst.
One of the ways to obtain acetic acid is from an oxidation reaction of acetaldehyde.
Acetic acid manipulation
Handling acetic acid should be done with caution. Depending on its concentration, it may carry more or less risks.
Due to its corrosive power, it can irritate the mucous membranes, cause damage to eyesight or burn the skin. It should always be handled with appropriate gloves to reduce the likelihood of injury.
At high temperatures, acetic acid becomes flammable . Even when mixed with air, it can cause an explosion . It should be mentioned, on the other hand, that it should be stored away from nitric acid, chromic acid, perchloric acid and ethylene glycol because it shows an incompatibility with these substances.
Although, like vinegar, acetic acid can be consumed without problems, a concentrated solution can be lethal since it is capable of modifying the acidity level of the blood .
Its use
The most common use of acetic acid is as vinegar , a product in which it is combined with water and used as a condiment . Vinegar is often used with oil in the preparation of salads, for example. In some cases, it is flavored with thyme, rosemary or tarragon, among other herbs.
Likewise, vinegar works as a preservative by slowing down the putrefaction processes of food. That is why it is used in pickles and marinades. Another use for vinegar appears in the production of dressings , such as hot sauces or ketchup.
The application of vinegar in cleaning tasks is also frequent. It helps remove stains from different surfaces and remove limescale from coffee makers and other appliances.
In the medical field, acetic acid is used as a dye for the detection of human papillomavirus. In histology, it serves to preserve tissues.
The surveying of photographs, the manufacture of photographic or cinematographic films and the production of cellophane and nylon are other processes that are carried out with acetic acid.
